
Growing adoption of gene synthesis market in the field of cancer vaccine research is anticipated to aid the total market at a CAGR of 27.9% by 2027, says Absolute Markets Insights.
Gene synthesis technology is being used by researchers to augment the currently available therapeutic approaches such as immunotherapy and chemotherapy. New therapeutic strategies such as ex vivo and in vivo cytokine gene transfer, drug sensitization with genes for prodrug approach and use of drug resistance genes for bone marrow protection from chemotherapy are some of the developments that aid the global gene synthesis industry.
Gene synthesis in the field of cancer will allow researchers to understand the roots of cancer and enable them to develop therapeutic treatments which can combat cancer at a cellular level. Thus this technology is widely used in cancer research studies from basic research on oncogenic signaling pathways, to design and development of DNA vaccines or therapeutic antibodies used for immuno-oncology treatments. In context to immune therapy, pharmaceutical giants are more focused on developing cancer vaccines through gene synthesis which will allow them to design antigens that can produce a desired immune response against the tumor cells but will not have an autoimmune response. Currently only two prophylactic cancer vaccine are approved by FDA for cervical cancer which are Cervarix by GSK and Gardasil by Merck. For therapeutic cancer vaccine, Sipuleucel-T (Provenge) is approved by FDA for prostate cancer. Companies such as GenScript are providing services and support for cancer research and development. The company aids gene synthesis of codon-optimized sequences to promote immune-stimulatory antigen expression. They have developed cutting edge technologies for custom design of powerful DNA vaccines.
Thus gene synthesis enables researchers to get a better understanding of cancer biology which can be applied in design and development of targeted antigens for cancer therapy, thus bringing in abundant potential for further market development.
“Living Beyond Cancer!! In May 2018, Legend Biotech, a subsidiary of GenScript announced FDA approval of their IND application on CAR-T immuno-cell therapy for the treatment of multiple myeloma. Legend biotech with its development partner Janssen biotech are to commence phase 1b/2 clinical trials for their LCAR-B38M CAR-T therapy. This is a dual epitope Chimeric Antigen Receptor T cell (CAR-T) immunotherapy product.”
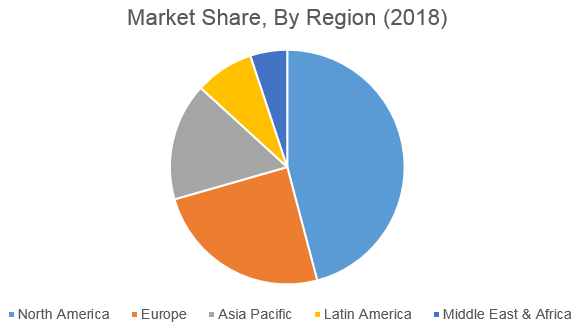
The detailed research study provides qualitative and quantitative analysis of the global gene synthesis market. The market has been analyzed from demand as well as supply side. The geographical analysis done emphasizes on each of the major countries across North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
Global Gene Synthesis Market Revenue, 2018
Some of the major players operating in the global gene synthesis market are GENEWIZ, Genscript, Integrated DNA Technologies, Inc, ATDBio Ltd, ATUM, BioCat GmbH, Biomatik, BIONEER CORPORATION, Blue Heron Biotech, LLC, Epoch Life Science Inc, Eurofins Genomics LLC, Synbio Technologies LLC, Thermo Fisher Scientific Inc, Vigene Biosciences Inc, Kaneka Eurogentec S.A., OriGene Technologies, Inc., ProteoGenix, Shanghai Medicilon inc., among others.
By Type
- Custom Gene Synthesis
- cDNA
- Customized Coding Sequences
- Genomic DNA
- RNAi constructs (shRNA, siRNA, miRNA)
- Others
- Gene Library Synthesis
By Method
- Oligo synthesis
- Phosphoramidite Reaction Cycle
- High-throughput Array-Based Gene Synthesis Technology
- Gene assembly
- Polymerase-Based
- Dual-Asymmetric (DA) PCR
- Overlap Extension (OE)
- Polymerase Cycling Assembly
- Thermodynamically-Balanced Inside-Out (TBIO)
- Microchip-based multiplex gene synthesis
- Others
- Ligase- Based
- Shotgun Ligation
- Two-Step Ligation and PCR
- Ligase Chain Reaction
- Brick-based
- Recombination-Based
- Sequence- and ligation -Independent Cloning (SLIC)
- Transformation-associated Recombination
- BioBrick assembly
- Polymerase-Based
By Components
- Software
- Services
By Application
- Research and Development
- Diagnosis
- Therapeutics
- Other applications
By End User
- Biotech and Pharmaceuticals Companies
- Academic and Research Institutes
- Contract Research Organizations
By Geography
- North America
- U.S.
- Canada
- Mexico
- Rest of North America
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Nordic Countries
- Benelux Union
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Southeast Asia
- Australia
- New Zealand
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America

