Cardiac Rhythm Management Devices Market By Product (Pacemakers (Implantable Pacemakers, External Pacemakers), Defibrillators (Implantable Cardioverter Defibrillators (ICD) (Subcutaneous ICD (S-ICD), Transvenous ICD (T-ICD), External Defibrillators (ED) (Manual ED, Automated ED (AED), Wearable Cardioverter Defibrillator), Cardiac Resynchronization Therapy (CRT) (CRT Defibrillators (CRT-D), CRT Pacemakers (CRT-P); By End User (Hospitals and Clinics, Home Settings, Ambulatory Surgical Centers); By Region (U.S., Rest of North America, France, UK, Germany, Spain, Italy, Rest of Europe, China, Japan, India, Southeast Asia, Rest of Asia Pacific, GCC Countries, Southern Africa, Rest of MEA, Brazil, Rest of Latin America) – Global Insights, Growth, Size, Comparative Analysis, Trends and Forecast, 2018 – 2026
Industry Trends
Cardiac Rhythm Management (CRM) is the treatment of arrhythmias, which are heart rhythm disorders. Arrhythmias cause dizzy spells, blackouts, palpitations and sudden cardiac arrest in patients. Cardiac Rhythm Management usually involves implanting of electronic devices such as defibrillators and pacemakers. Cardiac rhythm management devices are used to determine heart health and enable the physicians to offer a faster diagnosis. Various CRM devices are also being utilized to monitor patient activity and intracardiac pressures. The extensive use of cardiac rhythm management devices (CRMDs) is substantially improving the long-term prognosis and survival rates.
A consistent rise in instances of cardiovascular diseases is being observed, due to various lifestyle and food related disorders, positively impacting the demand for cardiac rhythm management devices. High cost of these devices, as well as a shortage of skilled professionals with expertise in cardiac monitoring has limited its growth in several emerging markets. Evolutionary advancements of these devices are enhancing functionality by improving their battery life and reduction in the device size.
Cardiac Rhythm Management Devices Market is expected to grow with a CAGR of 6.5% during the forecast period of 2018 to 2026. The study analyzes the market in terms of revenue and sales volume across all the major markets.
 Cardiac Rhythm Management Devices Market, by Product
Cardiac Rhythm Management Devices Market, by Product
Pacemakers (PM) are among the most commonly used cardiac rhythm management devices, which give electrical impulses to trigger the heartbeat when it is slow or stops altogether. An Implantable Cardioverter Defibrillator (ICD) automatically delivers a shock to restore the normal rhythm by detecting fast or chaotic beating of heart’s main pumping chamber, which often leads to sudden cardiac arrests. Most cardiac rhythm management devices also act as pacemakers and both these devices provide constant care to patients with irregular heart rhythms.
Cardiac Rhythm Management Devices Market, by Region
The significant presence of population requiring cardiac rhythm management therapy, due to the prevalence of serious risk factors such as diabetes, hypertension, obesity, and congestive heart failure across regions is influencing the demand of CRMDs in this market. Boston Scientific is a medical device manufacturer based in Marlborough, Massachusets, US, which markets a wide range of CRM devices. It is among the leading manufacturers of these products including RHYTHMIA HDx Mapping System that enables physicians to provide personalized care to patients suffering from arrhythmias. Biotronik is a company headquartered in Berlin, Germany and is gaining popularity for their CRT pacemaker with remote monitoring called Etrinsa 8 HF-T. The availability of such devices in the market catering to the specific requirements of cardiac patients is substantially improving cardiac care standards.
Competitive Landscape
The report provides both, qualitative and quantitative research of the cardiac rhythm management devices market, as well as provides worthy insights into the rational scenario and favored development methods adopted by the key contenders. The report also offers extensive research on the key players in this market and detailed insights on the competitiveness of these players. The key business strategies such as mergers and acquisitions (M&A), affiliations, collaborations, and contracts adopted by the major players are also recognized and analyzed in the report. For each company, the report recognizes their headquarter, competitors, product/service type, application and specification, pricing, and gross margin.
The Cardiac Rhythm Management Devices Market participants include Siemens AG, 3M Company(3M), Abiomed, Inc.(ABIOMED), Berlin Heart GmbH (Berlin Heart), Biotronik SE & Co. KG, Boston Scientific Corporation, Cardiac Science Corporation, General Electric Company (General Electric), Hill-Rom Services Inc., Jarvik Heart, Inc., Koninklijke Philips N.V., LivaNova PLC, Medtronic, NIHON KOHDEN CORPORATION, and ZOLL Medical Corporation amongst others.
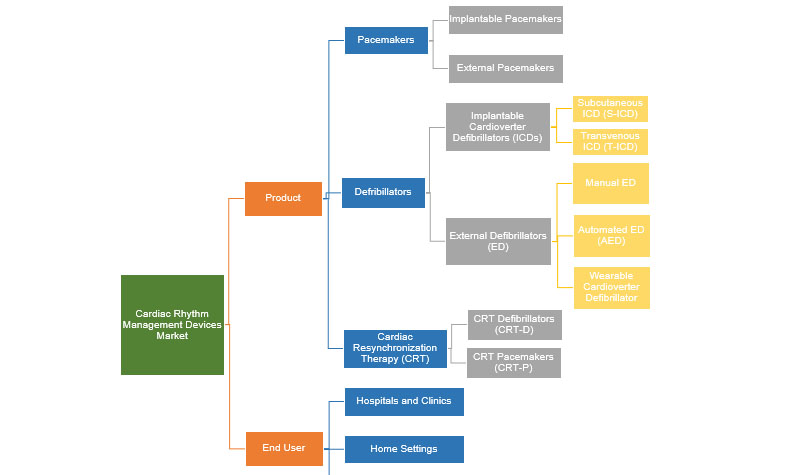
Table of Contents
![]()
1. Introduction
1.1. Market
Scope
1.2. Market
Segmentation
1.3. Methodology
1.4. Assumptions
2. Cardiac Rhythm Management Devices Market Snapshot
3. Executive Summary: Cardiac Rhythm Management Devices Market
4. Qualitative Analysis: Cardiac Rhythm Management Devices
Market
4.1. Introduction
4.1.1. Product
Definition
4.1.2. Industry
Development
4.2. Market
Dynamics
4.2.1. Drivers
4.2.2. Restraints
4.2.3. Opportunities
4.3. Trends in Cardiac Rhythm Management Devices Market
4.4. PESTLE Analysis
4.5. Porter’s Five Forces Analysis
4.6. SWOT Analysis
5. Global Cardiac Rhythm Management Devices Market Analysis and
Forecasts, 2018 – 2026
5.1. Overview
5.1.1. Global
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts
5.2. Global
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts, By
Product
5.2.1. Pacemakers
(Definition, Market Penetration, Market Revenue Expected to Increase by 2026,
Compound Annual Growth Rate (CAGR) and information on Implantable Pacemakers,
External Pacemakers)
5.2.1.1. Implantable Pacemakers
5.2.1.2. External Pacemakers
5.2.2. Defibrillators
(Definition, Market Penetration, Market Revenue Expected to Increase by 2026,
Compound Annual Growth Rate (CAGR) and information on Implantable Cardioverter
Defibrillators (ICDs), External Defibrillator (ED))
5.2.2.1. Implantable Cardioverter Defibrillators (ICDs)
5.2.2.2.
External Defibrillator
(ED)
5.2.3. Implantable
Cardioverter Defibrillators (ICDs) (Definition, Market Penetration, Market
Revenue Expected to Increase by 2026, Compound Annual Growth Rate (CAGR) and
information on Subcutaneous ICD (S-ICD), Transvenous ICD (T-ICD))
5.2.3.1.
Subcutaneous ICD (S-ICD)
5.2.3.2.
Transvenous ICD (T-ICD)
5.2.4. External
Defibrillator (ED) (Definition, Market Penetration, Market Revenue Expected to
Increase by 2026, Compound Annual Growth Rate (CAGR) and information on Manual
ED, Automated ED (AED), Wearable Cardioverter Defibrillator)
5.2.4.3.
Wearable Cardioverter
Defibrillator
5.2.5. Cardiac
Resynchronization Therapy (CRT) (Definition, Market Penetration, Market Revenue
Expected to Increase by 2026, Compound Annual Growth Rate (CAGR) and
information on CRT Defibrillators (CRT-D), CRT Pacemakers (CRT-P))
5.2.5.1. CRT Defibrillators (CRT-D)
5.2.5.2. CRT Pacemakers (CRT-P)
5.3. Key
Segment for Channeling Investments
5.3.1. By
Product
6. Global Cardiac Rhythm Management Devices Market Analysis and
Forecasts, 2018 – 2026
6.1. Overview
6.2. Global
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts, By End
User
6.2.1. Hospitals
and Clinics
6.2.1.1. Definition
6.2.1.2. Market Penetration
6.2.1.3. Market Revenue Expected to Increase by 2026
6.2.1.4. Compound Annual Growth Rate (CAGR)
6.2.2. Home
Settings
6.2.2.1. Definition
6.2.2.2. Market Penetration
6.2.2.3. Market Revenue Expected to Increase by 2026
6.2.2.4. Compound Annual Growth Rate (CAGR)
6.2.3. Ambulatory
Service Centers
6.2.3.1. Definition
6.2.3.2. Market Penetration
6.2.3.3. Market Revenue Expected to Increase by 2026
6.2.3.4. Compound Annual Growth Rate (CAGR)
6.3. Key
Segment for Channeling Investments
6.3.1. By End
User
7. North America Cardiac Rhythm Management Devices Market
Analysis and Forecasts, 2018 – 2026
7.1. Overview
7.1.1. North
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
7.2. North
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Product
7.2.1. Pacemakers
7.2.1.1. Implantable Pacemakers
7.2.1.2. External Pacemakers
7.2.2. Defibrillators
7.2.2.1.
Implantable Cardioverter
Defibrillators (ICDs)
7.2.2.1.1.
Subcutaneous ICD (S-ICD)
7.2.2.1.2.
Transvenous ICD (T-ICD)
7.2.2.2.
External Defibrillator
(ED)
7.2.2.2.2.
Automated ED (AED)
7.2.2.2.3.
Wearable Cardioverter Defibrillator
7.2.3. Cardiac
Resynchronization Therapy (CRT)
7.2.3.1. CRT Defibrillators (CRT-D)
7.2.3.2. CRT Pacemakers (CRT-P)
7.3. North
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By End User
7.3.1. Hospitals
and Clinics
7.3.2. Home Settings
7.3.3. Ambulatory
Service Centers
7.4. North
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Country
7.4.1. U.S.
7.4.1.1. U.S. Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
and Forecasts, By Product
7.4.1.1.1. Pacemakers
7.4.1.1.1.1. Implantable
Pacemakers
7.4.1.1.1.2. External
Pacemakers
7.4.1.1.2. Defibrillators
7.4.1.1.3.
Implantable Cardioverter
Defibrillators (ICDs)
7.4.1.1.3.1. Subcutaneous ICD (S-ICD)
7.4.1.1.3.2. Transvenous ICD (T-ICD)
7.4.1.1.4.
External Defibrillator
(ED)
7.4.1.1.4.1. Manual ED
7.4.1.1.4.2. Automated ED (AED)
7.4.1.1.4.3. Wearable Cardioverter Defibrillator
7.4.1.1.5. Cardiac Resynchronization Therapy (CRT)
7.4.1.1.5.1. CRT
Defibrillators (CRT-D)
7.4.1.1.5.2. CRT
Pacemakers (CRT-P)
7.4.1.2. U.S. Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
and Forecasts, By End User
7.4.1.2.1. Hospitals and Clinics
7.4.1.2.2. Home Settings
7.4.1.2.3. Ambulatory Service Centers
7.4.2. Rest of
North America
7.4.2.1. Rest of North America Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
7.4.2.1.1. Pacemakers
7.4.2.1.1.1. Implantable
Pacemakers
7.4.2.1.1.2. External
Pacemakers
7.4.2.1.2. Defibrillators
7.4.2.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
7.4.2.1.2.1.1.
Subcutaneous ICD (S-ICD)
7.4.2.1.2.1.2.
Transvenous ICD (T-ICD)
7.4.2.1.2.2. External Defibrillator (ED)
7.4.2.1.2.2.1.
Manual ED
7.4.2.1.2.2.2.
Automated ED (AED)
7.4.2.1.2.2.3.
Wearable Cardioverter
Defibrillator
7.4.2.1.3. Cardiac Resynchronization Therapy (CRT)
7.4.2.1.3.1. CRT
Defibrillators (CRT-D)
7.4.2.1.3.2. CRT
Pacemakers (CRT-P)
7.4.2.2. Rest of North America Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
7.4.2.2.1. Hospitals and Clinics
7.4.2.2.2. Home Settings
7.4.2.2.3. Ambulatory Service Centers
7.5. Key
Segment for Channeling Investments
7.5.1. By
Country
7.5.2. By
Product
7.5.3. By End
User
8. Europe Cardiac Rhythm Management Devices Market Analysis and
Forecasts, 2018 – 2026
8.1. Overview
8.1.1. Europe
Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
8.2. Europe
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts, By
Product
8.2.1. Pacemakers
8.2.1.1. Implantable Pacemakers
8.2.1.2. External Pacemakers
8.2.2. Defibrillators
8.2.2.1.
Implantable Cardioverter
Defibrillators (ICDs)
8.2.2.1.1.
Subcutaneous ICD (S-ICD)
8.2.2.1.2.
Transvenous ICD (T-ICD)
8.2.2.2.
External Defibrillator
(ED)
8.2.2.2.1.
Manual ED
8.2.2.2.2.
Automated ED (AED)
8.2.2.2.3.
Wearable Cardioverter
Defibrillator
8.2.3. Cardiac
Resynchronization Therapy (CRT)
8.2.3.1. CRT Defibrillators (CRT-D)
8.2.3.2. CRT Pacemakers (CRT-P)
8.3. Europe
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts, By End
User
8.3.1. Hospitals
and Clinics
8.3.2. Home
Settings
8.3.3. Ambulatory
Service Centers
8.4. Europe
Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and Forecasts, By
Country
8.4.1. France
8.4.1.1. France Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
8.4.1.1.1. Pacemakers
8.4.1.1.1.1. Implantable
Pacemakers
8.4.1.1.1.2. External
Pacemakers
8.4.1.1.2. Defibrillators
8.4.1.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.1.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.1.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.1.1.2.2. External Defibrillator (ED)
8.4.1.1.2.2.1.
Manual ED
8.4.1.1.2.2.2.
Automated ED (AED)
8.4.1.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.1.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.1.1.3.1. CRT
Defibrillators (CRT-D)
8.4.1.1.3.2. CRT
Pacemakers (CRT-P)
8.4.1.2. France Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
8.4.1.2.1. Hospitals and Clinics
8.4.1.2.2. Home Settings
8.4.1.2.3. Ambulatory Service Centers
8.4.2. The UK
8.4.2.1. The UK Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
8.4.2.1.1. Pacemakers
8.4.2.1.1.1. Implantable
Pacemakers
8.4.2.1.1.2. External
Pacemakers
8.4.2.1.2. Defibrillators
8.4.2.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.2.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.2.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.2.1.2.2. External Defibrillator (ED)
8.4.2.1.2.2.1.
Manual ED
8.4.2.1.2.2.2.
Automated ED (AED)
8.4.2.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.2.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.2.1.3.1. CRT
Defibrillators (CRT-D)
8.4.2.1.3.2. CRT
Pacemakers (CRT-P)
8.4.2.2. The UK Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
8.4.2.2.1. Hospitals and Clinics
8.4.2.2.2. Home Settings
8.4.2.2.3. Ambulatory Service Centers
8.4.3. Spain
8.4.3.1. Spain Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
8.4.3.1.1. Pacemakers
8.4.3.1.1.1. Implantable
Pacemakers
8.4.3.1.1.2. External
Pacemakers
8.4.3.1.2. Defibrillators
8.4.3.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.3.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.3.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.3.1.2.2. External Defibrillator (ED)
8.4.3.1.2.2.1.
Manual ED
8.4.3.1.2.2.2.
Automated ED (AED)
8.4.3.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.3.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.3.1.3.1. CRT
Defibrillators (CRT-D)
8.4.3.1.3.2. CRT
Pacemakers (CRT-P)
8.4.3.2. Spain Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
8.4.3.2.1. Hospitals and Clinics
8.4.3.2.2. Home Settings
8.4.3.2.3. Ambulatory Service Centers
8.4.4. Germany
8.4.4.1. Germany Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
8.4.4.1.1. Pacemakers
8.4.4.1.1.1. Implantable
Pacemakers
8.4.4.1.1.2. External
Pacemakers
8.4.4.1.2. Defibrillators
8.4.4.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.4.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.4.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.4.1.2.2. External Defibrillator (ED)
8.4.4.1.2.2.1.
Manual ED
8.4.4.1.2.2.2.
Automated ED (AED)
8.4.4.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.4.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.4.1.3.1. CRT
Defibrillators (CRT-D)
8.4.4.1.3.2. CRT
Pacemakers (CRT-P)
8.4.4.2. Germany Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
8.4.4.2.1. Hospitals and Clinics
8.4.4.2.2. Home Settings
8.4.4.2.3. Ambulatory Service Centers
8.4.5. Italy
8.4.5.1. Italy Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
8.4.5.1.1. Pacemakers
8.4.5.1.1.1. Implantable
Pacemakers
8.4.5.1.1.2. External
Pacemakers
8.4.5.1.2. Defibrillators
8.4.5.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.5.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.5.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.5.1.2.2. External Defibrillator (ED)
8.4.5.1.2.2.1.
Manual ED
8.4.5.1.2.2.2.
Automated ED (AED)
8.4.5.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.5.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.5.1.3.1. CRT
Defibrillators (CRT-D)
8.4.5.1.3.2. CRT
Pacemakers (CRT-P)
8.4.5.2. Italy Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
8.4.5.2.1. Hospitals and Clinics
8.4.5.2.2. Home Settings
8.4.5.2.3. Ambulatory Service Centers
8.4.6. Rest of
Europe
8.4.6.1. Rest of Europe Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
8.4.6.1.1. Pacemakers
8.4.6.1.1.1. Implantable
Pacemakers
8.4.6.1.1.2. External
Pacemakers
8.4.6.1.2. Defibrillators
8.4.6.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
8.4.6.1.2.1.1.
Subcutaneous ICD (S-ICD)
8.4.6.1.2.1.2.
Transvenous ICD (T-ICD)
8.4.6.1.2.2. External Defibrillator (ED)
8.4.6.1.2.2.1.
Manual ED
8.4.6.1.2.2.2.
Automated ED (AED)
8.4.6.1.2.2.3.
Wearable Cardioverter
Defibrillator
8.4.6.1.3. Cardiac Resynchronization Therapy (CRT)
8.4.6.1.3.1. CRT
Defibrillators (CRT-D)
8.4.6.1.3.2. CRT
Pacemakers (CRT-P)
8.4.6.2. Rest of Europe Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
8.4.6.2.1. Hospitals and Clinics
8.4.6.2.2. Home Settings
8.4.6.2.3. Ambulatory Service Centers
8.5. Key
Segment for Channeling Investments
8.5.1. By
Country
8.5.2. By
Product
8.5.3. By End
User
9. Asia Pacific Cardiac Rhythm Management Devices Market
Analysis and Forecasts, 2018 – 2026
9.1. Overview
9.1.1. Asia
Pacific Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
9.2. Asia
Pacific Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Product
9.2.1. Pacemakers
9.2.1.1. Implantable Pacemakers
9.2.1.2. External Pacemakers
9.2.2. Defibrillators
9.2.2.1.
Implantable Cardioverter
Defibrillators (ICDs)
9.2.2.1.1.
Subcutaneous ICD (S-ICD)
9.2.2.1.2.
Transvenous ICD (T-ICD)
9.2.2.2.
External Defibrillator
(ED)
9.2.2.2.1.
Manual ED
9.2.2.2.2.
Automated ED (AED)
9.2.2.2.3.
Wearable Cardioverter
Defibrillator
9.2.3. Cardiac
Resynchronization Therapy (CRT)
9.2.3.1. CRT Defibrillators (CRT-D)
9.2.3.2. CRT Pacemakers (CRT-P)
9.3. Asia
Pacific Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By End User
9.3.1. Hospitals
and Clinics
9.3.2. Home
Settings
9.3.3. Ambulatory
Service Centers
9.4. Asia
Pacific Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Country
9.4.1. China
9.4.1.1. China Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
9.4.1.1.1. Pacemakers
9.4.1.1.1.1. Implantable
Pacemakers
9.4.1.1.1.2. External
Pacemakers
9.4.1.1.2. Defibrillators
9.4.1.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
9.4.1.1.2.1.1.
Subcutaneous ICD (S-ICD)
9.4.1.1.2.1.2.
Transvenous ICD (T-ICD)
9.4.1.1.2.2. External Defibrillator (ED)
9.4.1.1.2.2.1.
Manual ED
9.4.1.1.2.2.2.
Automated ED (AED)
9.4.1.1.2.2.3.
Wearable Cardioverter
Defibrillator
9.4.1.1.3. Cardiac Resynchronization Therapy (CRT)
9.4.1.1.3.1. CRT
Defibrillators (CRT-D)
9.4.1.1.3.2. CRT
Pacemakers (CRT-P)
9.4.1.2. China Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
9.4.1.2.1. Hospitals and Clinics
9.4.1.2.2. Home Settings
9.4.1.2.3. Ambulatory Service Centers
9.4.2. Japan
9.4.2.1. Japan Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
9.4.2.1.1. Pacemakers
9.4.2.1.1.1. Implantable
Pacemakers
9.4.2.1.1.2. External
Pacemakers
9.4.2.1.2. Defibrillators
9.4.2.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
9.4.2.1.2.1.1.
Subcutaneous ICD (S-ICD)
9.4.2.1.2.1.2.
Transvenous ICD (T-ICD)
9.4.2.1.2.2. External Defibrillator (ED)
9.4.2.1.2.2.1.
Manual ED
9.4.2.1.2.2.2.
Automated ED (AED)
9.4.2.1.2.2.3.
Wearable Cardioverter
Defibrillator
9.4.2.1.3. Cardiac Resynchronization Therapy (CRT)
9.4.2.1.3.1. CRT
Defibrillators (CRT-D)
9.4.2.1.3.2. CRT
Pacemakers (CRT-P)
9.4.2.2. Japan Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
9.4.2.2.1. Hospitals and Clinics
9.4.2.2.2. Home Settings
9.4.2.2.3. Ambulatory Service Centers
9.4.3. India
9.4.3.1. India Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
9.4.3.1.1. Pacemakers
9.4.3.1.1.1. Implantable
Pacemakers
9.4.3.1.1.2. External
Pacemakers
9.4.3.1.2. Defibrillators
9.4.3.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
9.4.3.1.2.1.1.
Subcutaneous ICD (S-ICD)
9.4.3.1.2.1.2.
Transvenous ICD (T-ICD)
9.4.3.1.2.2. External Defibrillator (ED)
9.4.3.1.2.2.1.
Manual ED
9.4.3.1.2.2.2.
Automated ED (AED)
9.4.3.1.2.2.3.
Wearable Cardioverter
Defibrillator
9.4.3.1.3. Cardiac Resynchronization Therapy (CRT)
9.4.3.1.3.1. CRT
Defibrillators (CRT-D)
9.4.3.1.3.2. CRT
Pacemakers (CRT-P)
9.4.3.2. India Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
9.4.3.2.1. Hospitals and Clinics
9.4.3.2.2. Home Settings
9.4.3.2.3. Ambulatory Service Centers
9.4.4. Southeast
Asia
9.4.4.1. Southeast Asia Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
9.4.4.1.1. Pacemakers
9.4.4.1.1.1. Implantable
Pacemakers
9.4.4.1.1.2. External
Pacemakers
9.4.4.1.2. Defibrillators
9.4.4.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
9.4.4.1.2.1.1.
Subcutaneous ICD (S-ICD)
9.4.4.1.2.1.2.
Transvenous ICD (T-ICD)
9.4.4.1.2.2. External Defibrillator (ED)
9.4.4.1.2.2.1.
Manual ED
9.4.4.1.2.2.2.
Automated ED (AED)
9.4.4.1.2.2.3.
Wearable Cardioverter
Defibrillator
9.4.4.1.3. Cardiac Resynchronization Therapy (CRT)
9.4.4.1.3.1. CRT
Defibrillators (CRT-D)
9.4.4.1.3.2. CRT
Pacemakers (CRT-P)
9.4.4.2. Southeast Asia Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
9.4.4.2.1. Hospitals and Clinics
9.4.4.2.2. Home Settings
9.4.4.2.3. Ambulatory Service Centers
9.4.5. Rest of
Asia Pacific
9.4.5.1. Rest of Asia Pacific Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
9.4.5.1.1. Pacemakers
9.4.5.1.1.1. Implantable
Pacemakers
9.4.5.1.1.2. External
Pacemakers
9.4.5.1.2. Defibrillators
9.4.5.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
9.4.5.1.2.1.1.
Subcutaneous ICD (S-ICD)
9.4.5.1.2.1.2.
Transvenous ICD (T-ICD)
9.4.5.1.2.2. External Defibrillator (ED)
9.4.5.1.2.2.1.
Manual ED
9.4.5.1.2.2.2.
Automated ED (AED)
9.4.5.1.2.2.3.
Wearable Cardioverter
Defibrillator
9.4.5.1.3. Cardiac Resynchronization Therapy (CRT)
9.4.5.1.3.1. CRT
Defibrillators (CRT-D)
9.4.5.1.3.2. CRT
Pacemakers (CRT-P)
9.4.5.2. Rest of Asia Pacific Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
9.4.5.2.1. Hospitals and Clinics
9.4.5.2.2. Home Settings
9.4.5.2.3. Ambulatory Service Centers
9.5. Key
Segment for Channeling Investments
9.5.1. By
Country
9.5.2. By
Product
9.5.3. By End
User
10. Middle East and Africa Cardiac Rhythm Management Devices
Market Analysis and Forecasts, 2018 – 2026
10.1. Overview
10.1.1. Middle
East and Africa Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
10.2. Middle
East and Africa Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Product
10.2.1. Pacemakers
10.2.1.1. Implantable Pacemakers
10.2.1.2. External Pacemakers
10.2.2. Defibrillators
10.2.2.1.
Implantable Cardioverter
Defibrillators (ICDs)
10.2.2.1.1.
Subcutaneous ICD (S-ICD)
10.2.2.1.2.
Transvenous ICD (T-ICD)
10.2.2.2.
External Defibrillator
(ED)
10.2.2.2.1.
Manual ED
10.2.2.2.2.
Automated ED (AED)
10.2.2.2.3.
Wearable Cardioverter
Defibrillator
10.2.3. Cardiac
Resynchronization Therapy (CRT)
10.2.3.1. CRT Defibrillators (CRT-D)
10.2.3.2. CRT Pacemakers (CRT-P)
10.3. Middle
East and Africa Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By End User
10.3.1. Hospitals
and Clinics
10.3.2. Home
Settings
10.3.3. Ambulatory
Service Centers
10.4. Middle
East and Africa Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Country
10.4.1. GCC
Countries
10.4.1.1. GCC Countries Cardiac Rhythm Management Devices Market Revenue
(US$ Mn) and Forecasts, By Product
10.4.1.1.1. Pacemakers
10.4.1.1.1.1. Implantable
Pacemakers
10.4.1.1.1.2. External
Pacemakers
10.4.1.1.2. Defibrillators
10.4.1.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
10.4.1.1.2.1.1.
Subcutaneous ICD (S-ICD)
10.4.1.1.2.1.2.
Transvenous ICD (T-ICD)
10.4.1.1.2.2. External Defibrillator (ED)
10.4.1.1.2.2.1.
Manual ED
10.4.1.1.2.2.2.
Automated ED (AED)
10.4.1.1.2.2.3.
Wearable Cardioverter
Defibrillator
10.4.1.1.3. Cardiac Resynchronization Therapy (CRT)
10.4.1.1.3.1. CRT
Defibrillators (CRT-D)
10.4.1.1.3.2. CRT
Pacemakers (CRT-P)
10.4.1.2. GCC Countries Cardiac Rhythm Management Devices Market Revenue
(US$ Mn) and Forecasts, By End User
10.4.1.2.1. Hospitals and Clinics
10.4.1.2.2. Home Settings
10.4.1.2.3. Ambulatory Service Centers
10.4.2. Southern
Africa
10.4.2.1. Southern Africa Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
10.4.2.1.1. Pacemakers
10.4.2.1.1.1. Implantable
Pacemakers
10.4.2.1.1.2. External
Pacemakers
10.4.2.1.2. Defibrillators
10.4.2.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
10.4.2.1.2.1.1.
Subcutaneous ICD (S-ICD)
10.4.2.1.2.1.2.
Transvenous ICD (T-ICD)
10.4.2.1.2.2. External Defibrillator (ED)
10.4.2.1.2.2.1.
Manual ED
10.4.2.1.2.2.2.
Automated ED (AED)
10.4.2.1.2.2.3.
Wearable Cardioverter
Defibrillator
10.4.2.1.3. Cardiac Resynchronization Therapy (CRT)
10.4.2.1.3.1. CRT
Defibrillators (CRT-D)
10.4.2.1.3.2. CRT
Pacemakers (CRT-P)
10.4.2.2. Southern Africa Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
10.4.2.2.1. Hospitals and Clinics
10.4.2.2.2. Home Settings
10.4.2.2.3. Ambulatory Service Centers
10.4.3. Rest of
MEA
10.4.3.1. Rest of MEA Cardiac Rhythm Management Devices Market Revenue
(US$ Mn) and Forecasts, By Product
10.4.3.1.1. Pacemakers
10.4.3.1.1.1. Implantable
Pacemakers
10.4.3.1.1.2. External
Pacemakers
10.4.3.1.2. Defibrillators
10.4.3.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
10.4.3.1.2.1.1.
Subcutaneous ICD (S-ICD)
10.4.3.1.2.1.2.
Transvenous ICD (T-ICD)
10.4.3.1.2.2. External Defibrillator (ED)
10.4.3.1.2.2.1.
Manual ED
10.4.3.1.2.2.2.
Automated ED (AED)
10.4.3.1.2.2.3.
Wearable Cardioverter
Defibrillator
10.4.3.1.3. Cardiac Resynchronization Therapy (CRT)
10.4.3.1.3.1. CRT
Defibrillators (CRT-D)
10.4.3.1.3.2. CRT
Pacemakers (CRT-P)
10.4.3.2. Rest of MEA Cardiac Rhythm Management Devices Market Revenue
(US$ Mn) and Forecasts, By End User
10.4.3.2.1. Hospitals and Clinics
10.4.3.2.2. Home Settings
10.4.3.2.3. Ambulatory Service Centers
10.5. Key
Segment for Channeling Investments
10.5.1. By
Country
10.5.2. By
Product
10.5.3. By End
User
11. Latin America Cardiac Rhythm Management Devices Market
Analysis and Forecasts, 2018 – 2026
11.1. Overview
11.1.1. Latin
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn)
11.2. Latin
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Product
11.2.1. Pacemakers
11.2.1.1. Implantable Pacemakers
11.2.1.2. External Pacemakers
11.2.2. Defibrillators
11.2.2.1.
Implantable Cardioverter
Defibrillators (ICDs)
11.2.2.1.1.
Subcutaneous ICD (S-ICD)
11.2.2.1.2.
Transvenous ICD (T-ICD)
11.2.2.2.
External Defibrillator
(ED)
11.2.2.2.1.
Manual ED
11.2.2.2.2.
Automated ED (AED)
11.2.2.2.3.
Wearable Cardioverter
Defibrillator
11.2.3. Cardiac
Resynchronization Therapy (CRT)
11.2.3.1. CRT Defibrillators (CRT-D)
11.2.3.2. CRT Pacemakers (CRT-P)
11.3. Latin
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By End User
11.3.1. Hospitals
and Clinics
11.3.2. Home
Settings
11.3.3. Ambulatory
Service Centers
11.4. Latin
America Cardiac Rhythm Management Devices Market Revenue (US$ Mn) and
Forecasts, By Country
11.4.1. Brazil
11.4.1.1. Brazil Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By Product
11.4.1.1.1. Pacemakers
11.4.1.1.1.1. Implantable
Pacemakers
11.4.1.1.1.2. External
Pacemakers
11.4.1.1.2. Defibrillators
11.4.1.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
11.4.1.1.2.1.1.
Subcutaneous ICD (S-ICD)
11.4.1.1.2.1.2.
Transvenous ICD (T-ICD)
11.4.1.1.2.2. External Defibrillator (ED)
11.4.1.1.2.2.1.
Manual ED
11.4.1.1.2.2.2.
Automated ED (AED)
11.4.1.1.2.2.3.
Wearable Cardioverter
Defibrillator
11.4.1.1.3. Cardiac Resynchronization Therapy (CRT)
11.4.1.1.3.1. CRT
Defibrillators (CRT-D)
11.4.1.1.3.2. CRT
Pacemakers (CRT-P)
11.4.1.2. Brazil Cardiac Rhythm Management Devices Market Revenue (US$
Mn) and Forecasts, By End User
11.4.1.2.1. Hospitals and Clinics
11.4.1.2.2. Home Settings
11.4.1.2.3. Ambulatory Service Centers
11.4.2. Rest of
Latin America
11.4.2.1. Rest of Latin America Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By Product
11.4.2.1.1. Pacemakers
11.4.2.1.1.1. Implantable
Pacemakers
11.4.2.1.1.2. External
Pacemakers
11.4.2.1.2. Defibrillators
11.4.2.1.2.1. Implantable Cardioverter Defibrillators (ICDs)
11.4.2.1.2.1.1.
Subcutaneous ICD (S-ICD)
11.4.2.1.2.1.2.
Transvenous ICD (T-ICD)
11.4.2.1.2.2. External Defibrillator (ED)
11.4.2.1.2.2.1.
Manual ED
11.4.2.1.2.2.2.
Automated ED (AED)
11.4.2.1.2.2.3.
Wearable Cardioverter
Defibrillator
11.4.2.1.3. Cardiac Resynchronization Therapy (CRT)
11.4.2.1.3.1. CRT
Defibrillators (CRT-D)
11.4.2.1.3.2. CRT
Pacemakers (CRT-P)
11.4.2.2. Rest of Latin America Cardiac Rhythm Management Devices Market
Revenue (US$ Mn) and Forecasts, By End User
11.4.2.2.1. Hospitals and Clinics
11.4.2.2.2. Home Settings
11.4.2.2.3. Ambulatory Service Centers
11.5. Key
Segment for Channeling Investments
11.5.1. By
Country
11.5.2. By
Product
11.5.3. By End
User
12. Competitive Benchmarking
12.1. Player
Positioning Analysis
12.2. Global
Presence and Growth Strategies
13. Player Profiles
13.1. 3M
Company (3M)
13.1.1. Company
Details
13.1.2. Company
Overview
13.1.3. Product
Offerings
13.1.4. Key
Developments
13.1.5. Financial
Analysis
13.1.6. SWOT
Analysis
13.1.7. Business
Strategies
13.2. Abiomed,
Inc. (ABIOMED)
13.2.1. Company
Details
13.2.2. Company
Overview
13.2.3. Product
Offerings
13.2.4. Key
Developments
13.2.5. Financial
Analysis
13.2.6. SWOT
Analysis
13.2.7. Business
Strategies
13.3. Berlin
Heart GmbH (Berlin Heart)
13.3.1. Company
Details
13.3.2. Company
Overview
13.3.3. Product
Offerings
13.3.4. Key
Developments
13.3.5. Financial
Analysis
13.3.6. SWOT
Analysis
13.3.7. Business
Strategies
13.4. Biotronik
SE and Co. KG
13.4.1. Company
Details
13.4.2. Company
Overview
13.4.3. Product
Offerings
13.4.4. Key
Developments
13.4.5. Financial
Analysis
13.4.6. SWOT
Analysis
13.4.7. Business
Strategies
13.5. Boston
Scientific Corporation
13.5.1. Company
Details
13.5.2. Company
Overview
13.5.3. Product
Offerings
13.5.4. Key
Developments
13.5.5. Financial
Analysis
13.5.6. SWOT
Analysis
13.5.7. Business
Strategies
13.6. Cardiac
Science Corporation
13.6.1. Company
Details
13.6.2. Company
Overview
13.6.3. Product
Offerings
13.6.4. Key
Developments
13.6.5. Financial
Analysis
13.6.6. SWOT
Analysis
13.6.7. Business
Strategies
13.7. General
Electric Company (General Electric)
13.7.1. Company
Details
13.7.2. Company
Overview
13.7.3. Product
Offerings
13.7.4. Key
Developments
13.7.5. Financial
Analysis
13.7.6. SWOT
Analysis
13.7.7. Business
Strategies
13.8. Hill-Rom
Services Inc.
13.8.1. Company
Details
13.8.2. Company
Overview
13.8.3. Product
Offerings
13.8.4. Key
Developments
13.8.5. Financial
Analysis
13.8.6. SWOT
Analysis
13.8.7. Business
Strategies
13.9. Jarvik
Heart, Inc.
13.9.1. Company
Details
13.9.2. Company
Overview
13.9.3. Product
Offerings
13.9.4. Key
Developments
13.9.5. Financial
Analysis
13.9.6. SWOT
Analysis
13.9.7. Business
Strategies
13.10. Koninklijke
Philips N.V.
13.10.1. Company
Details
13.10.2. Company
Overview
13.10.3. Product
Offerings
13.10.4. Key
Developments
13.10.5. Financial
Analysis
13.10.6. SWOT
Analysis
13.10.7. Business
Strategies
13.11. LivaNova
PLC
13.11.1. Company
Details
13.11.2. Company
Overview
13.11.3. Product
Offerings
13.11.4. Key
Developments
13.11.5. Financial
Analysis
13.11.6. SWOT
Analysis
13.11.7. Business
Strategies
13.12. Medtronic
13.12.1. Company
Details
13.12.2. Company
Overview
13.12.3. Product
Offerings
13.12.4. Key
Developments
13.12.5. Financial
Analysis
13.12.6. SWOT
Analysis
13.12.7. Business
Strategies
13.13. NIHON
KOHDEN CORPORATION
13.13.1. Company
Details
13.13.2. Company
Overview
13.13.3. Product
Offerings
13.13.4. Key
Developments
13.13.5. Financial
Analysis
13.13.6. SWOT
Analysis
13.13.7. Business
Strategies
13.14. Siemens
AG
13.14.1. Company
Details
13.14.2. Company
Overview
13.14.3. Product
Offerings
13.14.4. Key
Developments
13.14.5. Financial
Analysis
13.14.6. SWOT
Analysis
13.14.7. Business
Strategies
13.15. ZOLL
Medical Corporation
13.15.1. Company
Details
13.15.2. Company
Overview
13.15.3. Product
Offerings
13.15.4. Key
Developments
13.15.5. Financial
Analysis
13.15.6. SWOT
Analysis
13.15.7. Business
Strategies
At Absolute Markets Insights, we are engaged in building both global as well as country specific reports. As a result, the approach taken for deriving the estimation and forecast for a specific country is a bit unique and different in comparison to the global research studies. In this case, we not only study the concerned market factors & trends prevailing in a particular country (from secondary research) but we also tend to calculate the actual market size & forecast from the revenue generated from the market participants involved in manufacturing or distributing the any concerned product. These companies can also be service providers. For analyzing any country specifically, we do consider the growth factors prevailing under the states/cities/county for the same. For instance, if we are analyzing an industry specific to United States, we primarily need to study about the states present under the same(where the product/service has the highest growth). Similar analysis will be followed by other countries. Our scope of the report changes with different markets.
Our research study is mainly implement through a mix of both secondary and primary research. Various sources such as industry magazines, trade journals, and government websites and trade associations are reviewed for gathering precise data. Primary interviews are conducted to validate the market size derived from secondary research. Industry experts, major manufacturers and distributors are contacted for further validation purpose on the current market penetration and growth trends.
Prominent participants in our primary research process include:
- Key Opinion Leaders namely the CEOs, CSOs, VPs, purchasing managers, amongst others
- Research and development participants, distributors/suppliers and subject matter experts
Secondary Research includes data extracted from paid data sources:
- Reuters
- Factiva
- Bloomberg
- One Source
- Hoovers
Research Methodology
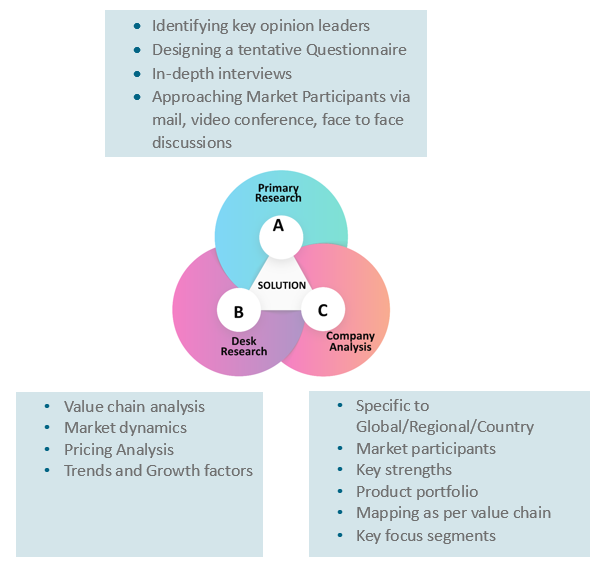
Key Inclusions

Reach to us
Call us on
+91-74002-42424
Drop us an email at
sales@absolutemarketsinsights.com
Why Absolute Markets Insights?
An effective strategy is the entity that influences a business to stand out of the crowd. An organization with a phenomenal strategy for success dependably has the edge over the rivals in the market. It offers the organizations a head start in planning their strategy. Absolute Market Insights is the new initiation in the industry that will furnish you with the lead your business needs. Absolute Market Insights is the best destination for your business intelligence and analytical solutions; essentially because our qualitative and quantitative sources of information are competent to give one-stop solutions. We inventively combine qualitative and quantitative research in accurate proportions to have the best report, which not only gives the most recent insights but also assists you to grow.