Bioanalytical Testing Services Market Size, Share & Trends Analysis Report By Molecule Type (Small Molecule, and Large Molecule), By Test Type (ADME, PK, PD, Bioavailability, Bioequivalence) - Global Opportunity Analysis and Industry Forecast, 2019-2026; By Region (U.S., Canada, Mexico, Rest of North America, France, UK, Germany, Spain, Italy, Nordic Countries, Benelux Union, Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia, Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of MEA, Brazil, Argentina, Rest of Latin America) – Global Insights, Growth, Size, Comparative Analysis, Trends and Forecast, 2019 - 2027
Industry Trends
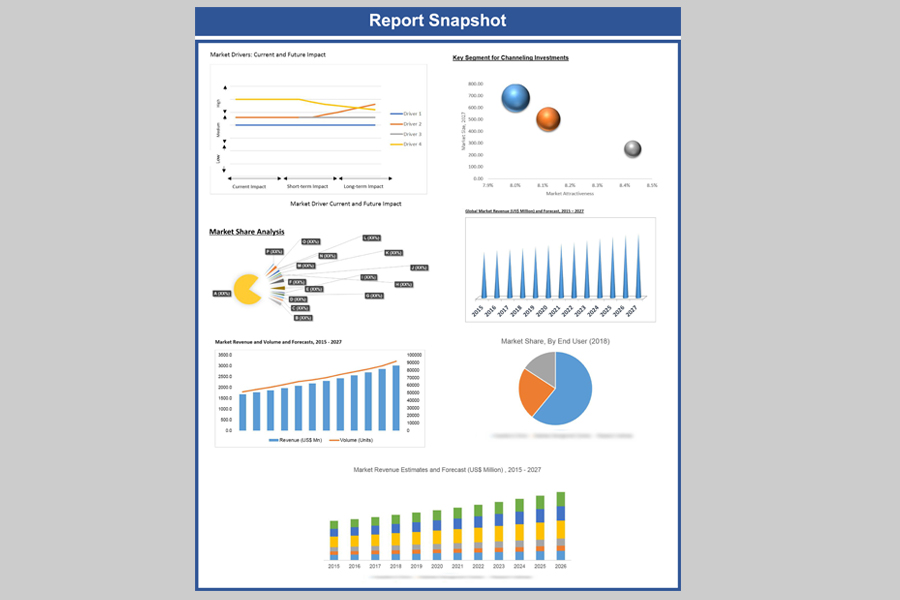
Global bioanalytical testing services market was valued at US$ 1743.80 million in 2018 and is expected to reach US$ 4387.01 million by 2027, growing at an estimated CAGR of 10.9% over the forecast period. Market growth can be attributed to increasing demand for analytical services for large-molecule drugs and biologics, number of biosimilar clinical trials, biopharmaceutical pipeline, approvals for biopharmaceuticals, and number of R&D. On the other hand, during the forecast period pricing pressures faced by major players and regulatory operation, cost and labor are expected to restrain the overall market growth to some extent.
Bioanalytical testing is the method used for the analysis of biotechnological or biological products. Bioanalytical tests are applied in drug discovery, drug development, and drug marketing. In recent years, increased focus on contract research development services by biopharmaceutical companies has created opportunities in the bioanalytical testing services market. Increasing frequency of outsourcing research and development activities by many biopharmaceutical companies to focus on their core competencies is expected to drive the expansion of the global bioanalytical testing services market during the forecast period. In addition, the cost-effectiveness associated with outsourcing services compared to in-house analysis is likely to drive the market even further. The strategy that key players in the pharmaceutical industry are implementing to face market competition is restocking their respective pipelines.
Few factors that are responsible for the growth of bioanalytical testing service are in the specific therapeutic areas of drug development and CRO (Contract Research Organization) that diversify their expertise compared to the pharma companies that also tends to perform clinical trials in broad geographies.
As there has been an increase in complexity as well as the number of standards associated with a single molecule, it may have to comply with the regulatory framework which is accelerating the growth in the outsourcing market of pharmaceutical analytical testing service. For companies to stay ahead in the competition they seek regulatory updates, look up for advisory services and also avail expertise so as to be in synchronization with evolving standards. Moreover, there are harmonized guidelines regularly provided by international organizations like the International Council for Harmonization (ICH), International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) amongst others.
There has also been an increase in demand for certain specific types of the test due to combination of molecules, development of biosimilars and other innovative medicines. For the companies it has become mandatory to follow local standards when these companies tries to diversify their business to new location.
The major challenge that is faced by the pharmaceuticals companies is the regulatory operations, cost and labor pressures. In order to procure new products and its approval and maintaining its compliance, manufacturers are struggling a lot, also while assuring the competitive cost of operation. The overall cost of operation has been increased due to high investments in regulatory information systems and labor, because of which pharmaceutical companies have decided to opt for outsourcing which is sustainable for research and development activities.
Demand for bioanalytical testing service is directly proportional to the innovation or new molecule development. Due to competitive pressure, lead-time to market and pricing concerns, the companies have outsourced bioanalytical testing service. The focus has moved to the rapid development of new molecules as the growing development is on customized care as well as on technological advancement have lowered the molecule lifecycle.
The demand from end users has influenced the performance of the market players in the pharmaceutical analytical testing domain. Consumers these days are more anxious about personal care, leading to higher consumption of pharmaceutical products, this is due to the increasing awareness level, improving accessibility, intense marketing efforts and government initiatives. Such consumption showing a rising trend is projected to drive the bioanalytical testing services market over the forecast period.
By Molecule Type Insights
Based on the molecule type the bioanalytical testing service market is divided into small molecule and large molecule. The small molecule segment is subjected to have a lucrative market over the next eight years. Most of the branded and generic drug compounds belong to small molecule category. With patent expiration, most of the generic manufacturers are required to conduct and submit the results of bioanalytical testing. Because of this factor, the segment is subjected to growth in the coming years and is in turn anticipated to aid the global bioanalytical testing services market.
The large molecule segment is anticipated to have healthy growth over the forecasted period. Large molecules are also referred to as biologics. They have therapeutic effects and are based on proteins. The large molecules are as heavy as 150kDa and are made up of several thousands of amino acids. Throughout the development process, the bioanalytical testing of large molecule drugs is needed. The sale of biologics has increased over the years exponentially and the pipeline for biologics is also rich.
Large molecule over the forecast year is expected to exhibit healthy growth. Advanced analytical instruments are needed for testing these molecules as well as to address the technical know-how. There is ample availability of testing infrastructure from bioanalytical service providers. Such a factor has aided the bioanalytical testing services market growth in recent past and is expected to continue the same trend over the forecast period
By Test Type Insights
Based on the test type, the market is further bifurcated into five types such as Absorption Distribution Metabolism Excretion (ADME), Bioavailability, Bioequivalence, Pharmacokinetics (PK) and Pharmacodynamics (PD).
Preclinical studies have made a progressive drug development from Pharmacokinetics (PK) and Pharmacodynamics (PD) analysis that exhibit rapid growth factors and are efficient in nature. As there has been a gradual evolution of drug development into sound pharmacology which has prompted several companies to broaden their service portfolio and to maintain their competitive edge in the market. This can be made evident from the presence of a large number of companies that have a broad service portfolio to serve various pharmaceutical development phases.
The category studies of bioavalilability and bioequivalence is attributed by small molecules of bioanalytical test services which has held a lucrative share in the forecasted period. This category has also attributed branded drug compounds and generic medicines which are largely adopted. Such factors are expected to assist the global biopharmaceutical analytical testing services market
The global bioanalytical testing services market in the year 2018 was stimulated by Bioavailability and Bioequivalence demand for complex, high-end pharmaceutical products and high reliability.
Regional Insights
There has been increasing investment from many developed countries with a further combination of several changes to the standard of clinical trial assessment.
North America was propelled to grow at a higher pace, this is because of the fact that the Original Equipment Manufacturers (OEMs) moved quickly towards electronics manufacturing service providers, as the volume of electronic components in the current pharmaceutical companies had increased effectively. Another reason for North America's market growth is the rapid increase in pharmaceutical manufacturers so as to meet the increasing demand for efficient healthcare. The government here has increased its support to reduce the healthcare cost, thereby the regional pharmaceutical outsourcing market is expected to grow further.
Amongst, major United States based drug manufacturers, a number of strategic alliances have been observed that offer PK investigation service in the region. North America held the largest share in 2018 and is anticipated to retain its dominancy over the future years. This is due to the presence of an effective regulatory structure pertaining to drug development processes of Pharmacokinetics (PK) and Pharmacodynamics (PD).
The market for Asia Pacific is expected to witness lucrative growth, due to the rising demand for outsourcing services which is in comparatively undeveloped markets. China, India, and Manila have been the delivery centres of outsourcing services in this region. For onshore clients and nearshore clients based out of Japan, China has been gaining popularity as a testing delivery location. Such a factor is expected to aid the global bioanalytical testing services market over the forecast period.
Biotechnology Testing Services Market Revenue & Forecast, (US$ Million), 2015 – 2027
Competitive Landscape
The report provides both, qualitative and quantitative research of bioanalytical testing services market, as well as provides comprehensive insights and development methods adopted by the key contenders. The key players present in the market are making collaborations and product launch strategies. The companies are also focusing on expanding their presence in the emerging markets. For each company, the report studies their global presence, competitors, service offerings and specification amongst others.
The global bioanalytical testing services market includes some of the major players such as Charles River Laboratories International Inc. (U.S.), BioReliance Corporation (U.S.), WuXi PharmaTech (Cayman) Inc. (China), Bioclin Research Laboratories (Ireland), Eurofins Scientific SE (Luxembourg), SGS (Switzerland), LabCorp (U.S.), PPD Inc. (U.S.), Intertek Group Plc. (U.K.) and PRA Health Sciences (U.S.).
Bioanalytical testing services Market:
- By Molecule type
- Small Molecule
- Large Molecule
- By Test type
- ADME
- PK
- PD
- Bioavailability
- Bioequivalence
- By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Nordic Countries
- Denmark
- Finland
- Iceland
- Sweden
- Norway
- Benelux Union
- Belgium
- The Netherlands
- Luxembourg
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Southeast Asia
- Indonesia
- Thailand
- Malaysia
- Singapore
- Rest of Southeast Asia
- Rest of Asia Pacific
- Middle East and Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- North America
Table of Contents
![]()
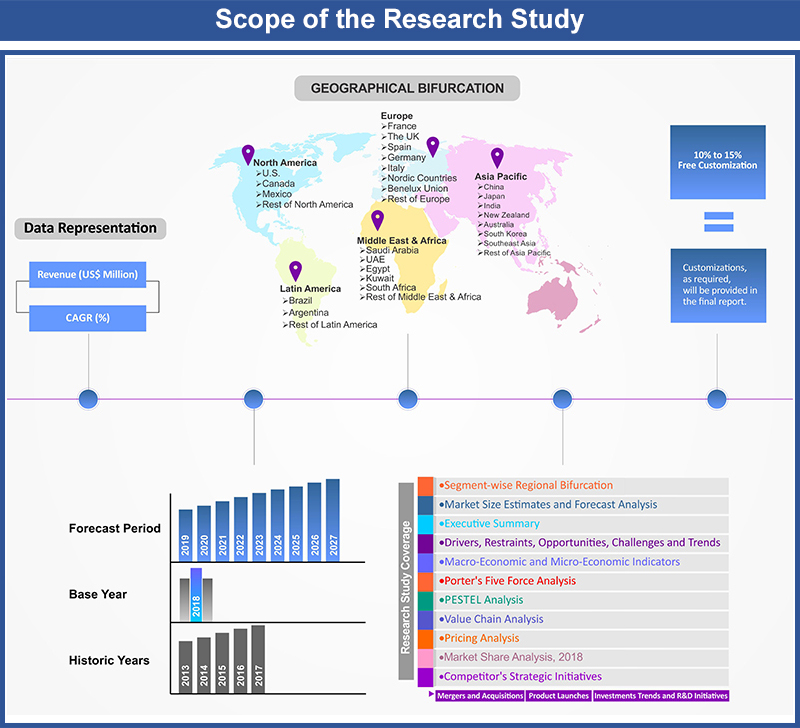
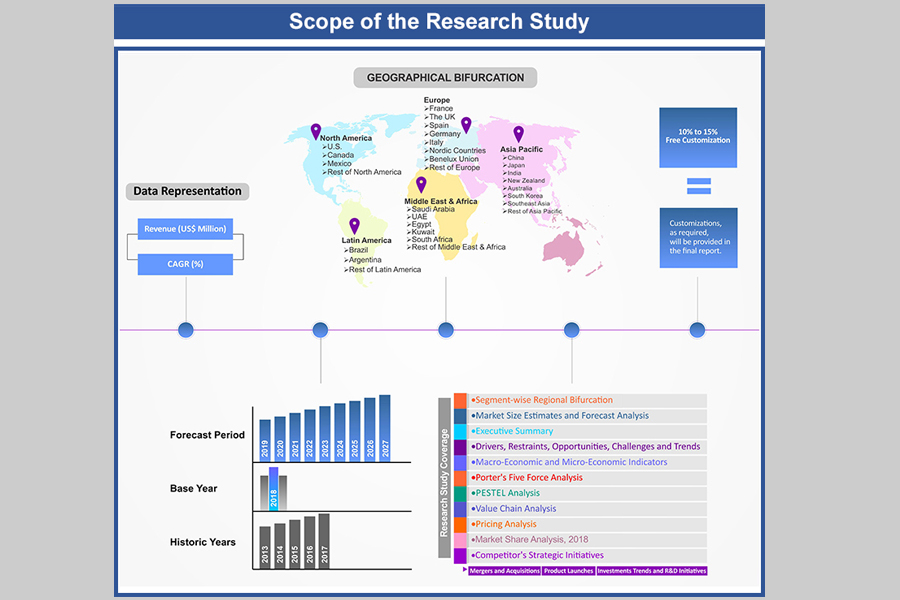
1. Market Scope
1.1. Market
Segmentation
1.2. Years
Considered
1.2.1. Historic
Years: 2015 - 2017
1.2.2. Base
Year: 2018
1.2.3. Forecast
Years: 2019 – 2027
2. Key Target Audiences
3. Research Methodology
3.1. Primary
Research
3.1.1. Research
Questionnaire
3.1.2. Global
Percentage Breakdown
3.1.3. Primary
Interviews: Key Opinion Leaders (KOLs)
3.2. Secondary
Research
3.2.1. Paid
Databases
3.2.2. Secondary
Sources
3.3. Market
Size Estimates
3.3.1. Top-Down
Approach
3.3.2. Bottom-Up
Approach
3.4. Data
Triangulation Methodology
3.5. Research
Assumptions
4. Recommendations and Insights from AMI’s Perspective**
5. Holistic Overview of Bioanalytical testing services Market
6. Market Synopsis:
Bioanalytical testing services Market
7. Bioanalytical testing services Market Analysis: Qualitative
Perspective
7.1. Introduction
7.1.1. Product
Definition
7.1.2. Industry
Development
7.2. Market
Dynamics
7.2.1. Drivers
7.2.2. Restraints
7.2.3. Opportunities
7.3. Trends in
Bioanalytical testing services Market
7.4. Market
Determinants Radar Chart
7.5. Macro-Economic
and Micro-Economic Indicators: Bioanalytical testing services Market
7.6. Porter’s
Five Force Analysis
8. Global Bioanalytical testing services Market Analysis and
Forecasts, 2019 – 2027
8.1. Overview
8.1.1. Global
Bioanalytical testing services Market Revenue (US$ Mn)
8.2. Global
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
8.2.1. Small
Molecule
8.2.1.1. Definition
8.2.1.2. Market
Estimation and Penetration, 2015 – 2018
8.2.1.3. Market
Forecast, 2019 – 2027
8.2.1.4. Compound
Annual Growth Rate (CAGR)
8.2.1.5. Regional
Bifurcation
8.2.1.5.1. North
America
8.2.1.5.1.1. Market
Estimation, 2015 – 2018
8.2.1.5.1.2. Market
Forecast, 2019 – 2027
8.2.1.5.2. Europe
8.2.1.5.2.1. Market
Estimation, 2015 – 2018
8.2.1.5.2.2. Market
Forecast, 2019 – 2027
8.2.1.5.3. Asia
Pacific
8.2.1.5.3.1. Market
Estimation, 2015 – 2018
8.2.1.5.3.2. Market
Forecast, 2019 – 2027
8.2.1.5.4. Middle
East and Africa
8.2.1.5.4.1. Market
Estimation, 2015 – 2018
8.2.1.5.4.2. Market
Forecast, 2019 – 2027
8.2.1.5.5. Latin
America
8.2.1.5.5.1. Market
Estimation, 2015 – 2018
8.2.1.5.5.2. Market
Forecast, 2019 – 2027
8.2.2. Large
Molecule
8.2.2.1. Definition
8.2.2.2. Market
Estimation and Penetration, 2015 – 2018
8.2.2.3. Market
Forecast, 2019 – 2027
8.2.2.4. Compound
Annual Growth Rate (CAGR)
8.2.2.5. Regional
Bifurcation
8.2.2.5.1. North
America
8.2.2.5.1.1. Market
Estimation, 2015 – 2018
8.2.2.5.1.2. Market
Forecast, 2019 – 2027
8.2.2.5.2. Europe
8.2.2.5.2.1. Market
Estimation, 2015 – 2018
8.2.2.5.2.2. Market
Forecast, 2019 – 2027
8.2.2.5.3. Asia
Pacific
8.2.2.5.3.1. Market
Estimation, 2015 – 2018
8.2.2.5.3.2. Market
Forecast, 2019 – 2027
8.2.2.5.4. Middle
East and Africa
8.2.2.5.4.1. Market
Estimation, 2015 – 2018
8.2.2.5.4.2. Market
Forecast, 2019 – 2027
8.2.2.5.5. Latin
America
8.2.2.5.5.1. Market
Estimation, 2015 – 2018
8.2.2.5.5.2. Market
Forecast, 2019 – 2027
8.3. Key
Segment for Channeling Investments
8.3.1. By
Molecule type
9. Global Bioanalytical testing services Market Analysis and
Forecasts, 2019 – 2027
9.1. Overview
9.2. Global
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
9.2.1. ADME
9.2.1.1. Definition
9.2.1.2. Market
Estimation and Penetration, 2015 – 2018
9.2.1.3. Market
Forecast, 2019 – 2027
9.2.1.4. Compound
Annual Growth Rate (CAGR)
9.2.1.5. Regional
Bifurcation
9.2.1.5.1. North
America
9.2.1.5.1.1. Market
Estimation, 2015 – 2018
9.2.1.5.1.2. Market
Forecast, 2019 – 2027
9.2.1.5.2. Europe
9.2.1.5.2.1. Market
Estimation, 2015 – 2018
9.2.1.5.2.2. Market
Forecast, 2019 – 2027
9.2.1.5.3. Asia
Pacific
9.2.1.5.3.1. Market
Estimation, 2015 – 2018
9.2.1.5.3.2. Market
Forecast, 2019 – 2027
9.2.1.5.4. Middle
East and Africa
9.2.1.5.4.1. Market
Estimation, 2015 – 2018
9.2.1.5.4.2. Market
Forecast, 2019 – 2027
9.2.1.5.5. Latin
America
9.2.1.5.5.1. Market
Estimation, 2015 – 2018
9.2.1.5.5.2. Market
Forecast, 2019 – 2027
9.2.2. PK
9.2.2.1. Definition
9.2.2.2. Market
Estimation and Penetration, 2015 – 2018
9.2.2.3. Market
Forecast, 2019 – 2027
9.2.2.4. Compound
Annual Growth Rate (CAGR)
9.2.2.5. Regional
Bifurcation
9.2.2.5.1. North
America
9.2.2.5.1.1. Market
Estimation, 2015 – 2018
9.2.2.5.1.2. Market
Forecast, 2019 – 2027
9.2.2.5.2. Europe
9.2.2.5.2.1. Market
Estimation, 2015 – 2018
9.2.2.5.2.2. Market
Forecast, 2019 – 2027
9.2.2.5.3. Asia
Pacific
9.2.2.5.3.1. Market
Estimation, 2015 – 2018
9.2.2.5.3.2. Market
Forecast, 2019 – 2027
9.2.2.5.4. Middle
East and Africa
9.2.2.5.4.1. Market
Estimation, 2015 – 2018
9.2.2.5.4.2. Market
Forecast, 2019 – 2027
9.2.2.5.5. Latin
America
9.2.2.5.5.1. Market
Estimation, 2015 – 2018
9.2.2.5.5.2. Market
Forecast, 2019 – 2027
9.2.3. PD
9.2.3.1. Definition
9.2.3.2. Market
Estimation and Penetration, 2015 – 2018
9.2.3.3. Market
Forecast, 2019 – 2027
9.2.3.4. Compound
Annual Growth Rate (CAGR)
9.2.3.5. Regional
Bifurcation
9.2.3.5.1. North
America
9.2.3.5.1.1. Market
Estimation, 2015 – 2018
9.2.3.5.1.2. Market
Forecast, 2019 – 2027
9.2.3.5.2. Europe
9.2.3.5.2.1. Market
Estimation, 2015 – 2018
9.2.3.5.2.2. Market
Forecast, 2019 – 2027
9.2.3.5.3. Asia
Pacific
9.2.3.5.3.1. Market
Estimation, 2015 – 2018
9.2.3.5.3.2. Market
Forecast, 2019 – 2027
9.2.3.5.4. Middle
East and Africa
9.2.3.5.4.1. Market
Estimation, 2015 – 2018
9.2.3.5.4.2. Market
Forecast, 2019 – 2027
9.2.3.5.5. Latin
America
9.2.3.5.5.1. Market
Estimation, 2015 – 2018
9.2.3.5.5.2. Market
Forecast, 2019 – 2027
9.2.4. Bioavailability
9.2.4.1. Definition
9.2.4.2. Market
Estimation and Penetration, 2015 – 2018
9.2.4.3. Market
Forecast, 2019 – 2027
9.2.4.4. Compound
Annual Growth Rate (CAGR)
9.2.4.5. Regional
Bifurcation
9.2.4.5.1. North
America
9.2.4.5.1.1. Market
Estimation, 2015 – 2018
9.2.4.5.1.2. Market
Forecast, 2019 – 2027
9.2.4.5.2. Europe
9.2.4.5.2.1. Market
Estimation, 2015 – 2018
9.2.4.5.2.2. Market
Forecast, 2019 – 2027
9.2.4.5.3. Asia
Pacific
9.2.4.5.3.1. Market
Estimation, 2015 – 2018
9.2.4.5.3.2. Market
Forecast, 2019 – 2027
9.2.4.5.4. Middle
East and Africa
9.2.4.5.4.1. Market
Estimation, 2015 – 2018
9.2.4.5.4.2. Market
Forecast, 2019 – 2027
9.2.4.5.5. Latin
America
9.2.4.5.5.1. Market
Estimation, 2015 – 2018
9.2.4.5.5.2. Market
Forecast, 2019 – 2027
9.2.5. Bioequivalence
9.2.5.1. Definition
9.2.5.2. Market
Estimation and Penetration, 2015 – 2018
9.2.5.3. Market
Forecast, 2019 – 2027
9.2.5.4. Compound
Annual Growth Rate (CAGR)
9.2.5.5. Regional
Bifurcation
9.2.5.5.1. North
America
9.2.5.5.1.1. Market
Estimation, 2015 – 2018
9.2.5.5.1.2. Market
Forecast, 2019 – 2027
9.2.5.5.2. Europe
9.2.5.5.2.1. Market
Estimation, 2015 – 2018
9.2.5.5.2.2. Market
Forecast, 2019 – 2027
9.2.5.5.3. Asia
Pacific
9.2.5.5.3.1. Market
Estimation, 2015 – 2018
9.2.5.5.3.2. Market
Forecast, 2019 – 2027
9.2.5.5.4. Middle
East and Africa
9.2.5.5.4.1. Market
Estimation, 2015 – 2018
9.2.5.5.4.2. Market
Forecast, 2019 – 2027
9.2.5.5.5. Latin
America
9.2.5.5.5.1. Market
Estimation, 2015 – 2018
9.2.5.5.5.2. Market
Forecast, 2019 – 2027
9.3. Key
Segment for Channeling Investments
9.3.1. By Test
type
10. North America Bioanalytical testing services Market Analysis
and Forecasts, 2019 - 2027
10.1. Overview
10.1.1. North
America Bioanalytical testing services Market Revenue (US$ Mn)
10.2. North
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
10.2.1. Small
Molecule
10.2.2. Large
Molecule
10.3. North
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
10.3.1. ADME
10.3.2. PK
10.3.3. PD
10.3.4. Bioavailability
10.3.5. Bioequivalence
10.4. North
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Country
10.4.1. U.S
10.4.1.1. U.S
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
10.4.1.1.1. Small
Molecule
10.4.1.1.2. Large
Molecule
10.4.1.2. U.S
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
10.4.1.2.1. ADME
10.4.1.2.2. PK
10.4.1.2.3. PD
10.4.1.2.4. Bioavailability
10.4.1.2.5. Bioequivalence
10.4.2. Canada
10.4.2.1. Canada
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
10.4.2.1.1. Small
Molecule
10.4.2.1.2. Large
Molecule
10.4.2.2. Canada
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
10.4.2.2.1. ADME
10.4.2.2.2. PK
10.4.2.2.3. PD
10.4.2.2.4. Bioavailability
10.4.2.2.5. Bioequivalence
10.4.3. Mexico
10.4.3.1. Mexico
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
10.4.3.1.1. Small
Molecule
10.4.3.1.2. Large
Molecule
10.4.3.2. Mexico
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
10.4.3.2.1. ADME
10.4.3.2.2. PK
10.4.3.2.3. PD
10.4.3.2.4. Bioavailability
10.4.3.2.5. Bioequivalence
10.4.4. Rest of
North America
10.4.4.1. Rest
of North America Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Molecule type
10.4.4.1.1. Small
Molecule
10.4.4.1.2. Large
Molecule
10.4.4.2. Rest
of North America Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Test type
10.4.4.2.1. ADME
10.4.4.2.2. PK
10.4.4.2.3. PD
10.4.4.2.4. Bioavailability
10.4.4.2.5. Bioequivalence
10.5. Key
Segment for Channeling Investments
10.5.1. By
Country
10.5.2. By
Molecule type
10.5.3. By Test
type
11. Europe Bioanalytical testing services Market Analysis and
Forecasts, 2019 - 2027
11.1. Overview
11.1.1. Europe
Bioanalytical testing services Market Revenue (US$ Mn)
11.2. Europe
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.2.1. Small
Molecule
11.2.2. Large
Molecule
11.3. Europe
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
11.3.1. ADME
11.3.2. PK
11.3.3. PD
11.3.4. Bioavailability
11.3.5. Bioequivalence
11.4. Europe
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Country
11.4.1. France
11.4.1.1. France
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.1.1.1. Small
Molecule
11.4.1.1.2. Large
Molecule
11.4.1.2. France
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
11.4.1.2.1. ADME
11.4.1.2.2. PK
11.4.1.2.3. PD
11.4.1.2.4. Bioavailability
11.4.1.2.5. Bioequivalence
11.4.2. The UK
11.4.2.1. The UK
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.2.1.1. Small
Molecule
11.4.2.1.2. Large
Molecule
11.4.2.2. The
UK Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
11.4.2.2.1. ADME
11.4.2.2.2. PK
11.4.2.2.3. PD
11.4.2.2.4. Bioavailability
11.4.2.2.5. Bioequivalence
11.4.3. Spain
11.4.3.1. Spain
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.3.1.1. Small
Molecule
11.4.3.1.2. Large
Molecule
11.4.3.2. Spain
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
11.4.3.2.1. ADME
11.4.3.2.2. PK
11.4.3.2.3. PD
11.4.3.2.4. Bioavailability
11.4.3.2.5. Bioequivalence
11.4.4. Germany
11.4.4.1. Germany
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.4.1.1. Small
Molecule
11.4.4.1.2. Large
Molecule
11.4.4.2. Germany
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
11.4.4.2.1. ADME
11.4.4.2.2. PK
11.4.4.2.3. PD
11.4.4.2.4. Bioavailability
11.4.4.2.5. Bioequivalence
11.4.5. Italy
11.4.5.1. Italy
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.5.1.1. Small
Molecule
11.4.5.1.2. Large
Molecule
11.4.5.2. Italy
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
11.4.5.2.1. ADME
11.4.5.2.2. PK
11.4.5.2.3. PD
11.4.5.2.4. Bioavailability
11.4.5.2.5. Bioequivalence
11.4.6. Nordic
Countries
11.4.6.1. Nordic
Countries Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
11.4.6.1.1. Small
Molecule
11.4.6.1.2. Large
Molecule
11.4.6.2. Nordic
Countries Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
11.4.6.2.1. ADME
11.4.6.2.2. PK
11.4.6.2.3. PD
11.4.6.2.4. Bioavailability
11.4.6.2.5. Bioequivalence
11.4.6.3. Nordic
Countries Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Country
11.4.6.3.1. Denmark
11.4.6.3.2. Finland
11.4.6.3.3. Iceland
11.4.6.3.4. Sweden
11.4.6.3.5. Norway
11.4.7. Benelux
Union
11.4.7.1. Benelux
Union Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
11.4.7.1.1. Small
Molecule
11.4.7.1.2. Large
Molecule
11.4.7.2. Benelux
Union Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
11.4.7.2.1. ADME
11.4.7.2.2. PK
11.4.7.2.3. PD
11.4.7.2.4. Bioavailability
11.4.7.2.5. Bioequivalence
11.4.7.3. Benelux
Union Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Country
11.4.7.3.1. Belgium
11.4.7.3.2. The
Netherlands
11.4.7.3.3. Luxembourg
11.4.8. Rest of
Europe
11.4.8.1. Rest
of Europe Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
11.4.8.1.1. Small
Molecule
11.4.8.1.2. Large
Molecule
11.4.8.2. Rest
of Europe Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
11.4.8.2.1. ADME
11.4.8.2.2. PK
11.4.8.2.3. PD
11.4.8.2.4. Bioavailability
11.4.8.2.5. Bioequivalence
11.5. Key
Segment for Channeling Investments
11.5.1. By
Country
11.5.2. By
Molecule type
11.5.3. By Test
type
12. Asia Pacific Bioanalytical testing services Market Analysis
and Forecasts, 2019 - 2027
12.1. Overview
12.1.1. Asia
Pacific Bioanalytical testing services Market Revenue (US$ Mn)
12.2. Asia
Pacific Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
12.2.1. Small
Molecule
12.2.2. Large
Molecule
12.3. Asia
Pacific Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
12.3.1. ADME
12.3.2. PK
12.3.3. PD
12.3.4. Bioavailability
12.3.5. Bioequivalence
12.4. Asia
Pacific Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Country
12.4.1. China
12.4.1.1. China
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.1.1.1. Small
Molecule
12.4.1.1.2. Large
Molecule
12.4.1.2. China
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
12.4.1.2.1. ADME
12.4.1.2.2. PK
12.4.1.2.3. PD
12.4.1.2.4. Bioavailability
12.4.1.2.5. Bioequivalence
12.4.2. Japan
12.4.2.1. Japan
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.2.1.1. Small
Molecule
12.4.2.1.2. Large
Molecule
12.4.2.2. Japan
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
12.4.2.2.1. ADME
12.4.2.2.2. PK
12.4.2.2.3. PD
12.4.2.2.4. Bioavailability
12.4.2.2.5. Bioequivalence
12.4.3. India
12.4.3.1. India
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.3.1.1. Small
Molecule
12.4.3.1.2. Large
Molecule
12.4.3.2. India
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
12.4.3.2.1. ADME
12.4.3.2.2. PK
12.4.3.2.3. PD
12.4.3.2.4. Bioavailability
12.4.3.2.5. Bioequivalence
12.4.4. New
Zealand
12.4.4.1. New
Zealand Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
12.4.4.1.1. Small
Molecule
12.4.4.1.2. Large
Molecule
12.4.4.2. New
Zealand Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
12.4.4.2.1. ADME
12.4.4.2.2. PK
12.4.4.2.3. PD
12.4.4.2.4. Bioavailability
12.4.4.2.5. Bioequivalence
12.4.5. Australia
12.4.5.1. Australia
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.5.1.1. Small
Molecule
12.4.5.1.2. Large
Molecule
12.4.5.2. Australia
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
12.4.5.2.1. ADME
12.4.5.2.2. PK
12.4.5.2.3. PD
12.4.5.2.4. Bioavailability
12.4.5.2.5. Bioequivalence
12.4.6. South
Korea
12.4.6.1. South
Korea Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.6.1.1. Small
Molecule
12.4.6.1.2. Large
Molecule
12.4.6.2. South
Korea Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
12.4.6.2.1. ADME
12.4.6.2.2. PK
12.4.6.2.3. PD
12.4.6.2.4. Bioavailability
12.4.6.2.5. Bioequivalence
12.4.7. Southeast
Asia
12.4.7.1. Southeast
Asia Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
12.4.7.1.1. Small
Molecule
12.4.7.1.2. Large
Molecule
12.4.7.2. Southeast
Asia Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
12.4.7.2.1. ADME
12.4.7.2.2. PK
12.4.7.2.3. PD
12.4.7.2.4. Bioavailability
12.4.7.2.5. Bioequivalence
12.4.7.3. Southeast
Asia Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Country
12.4.7.3.1. Indonesia
12.4.7.3.2. Thailand
12.4.7.3.3. Malaysia
12.4.7.3.4. Singapore
12.4.7.3.5. Rest
of Southeast Asia
12.4.8. Rest of
Asia Pacific
12.4.8.1. Rest
of Asia Pacific Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Molecule type
12.4.8.1.1. Small
Molecule
12.4.8.1.2. Large
Molecule
12.4.8.2. Rest
of Asia Pacific Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
12.4.8.2.1. ADME
12.4.8.2.2. PK
12.4.8.2.3. PD
12.4.8.2.4. Bioavailability
12.4.8.2.5. Bioequivalence
12.5. Key
Segment for Channeling Investments
12.5.1. By
Country
12.5.2. By
Molecule type
12.5.3. By Test
type
13. Middle East and Africa Bioanalytical testing services Market
Analysis and Forecasts, 2019 - 2027
13.1. Overview
13.1.1. Middle
East and Africa Bioanalytical testing services Market Revenue (US$ Mn)
13.2. Middle
East and Africa Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Molecule type
13.2.1. Small
Molecule
13.2.2. Large
Molecule
13.3. Middle
East and Africa Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Test type
13.3.1. ADME
13.3.2. PK
13.3.3. PD
13.3.4. Bioavailability
13.3.5. Bioequivalence
13.4. Middle
East and Africa Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Country
13.4.1. Saudi
Arabia
13.4.1.1. Saudi
Arabia Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
13.4.1.1.1. Small
Molecule
13.4.1.1.2. Large
Molecule
13.4.1.2. Saudi
Arabia Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
13.4.1.2.1. ADME
13.4.1.2.2. PK
13.4.1.2.3. PD
13.4.1.2.4. Bioavailability
13.4.1.2.5. Bioequivalence
13.4.2. UAE
13.4.2.1. UAE Bioanalytical
testing services Market Revenue (US$ Mn) and Forecasts, By Molecule type
13.4.2.1.1. Small
Molecule
13.4.2.1.2. Large
Molecule
13.4.2.2. UAE
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
13.4.2.2.1. ADME
13.4.2.2.2. PK
13.4.2.2.3. PD
13.4.2.2.4. Bioavailability
13.4.2.2.5. Bioequivalence
13.4.3. Egypt
13.4.3.1. Egypt
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
13.4.3.1.1. Small
Molecule
13.4.3.1.2. Large
Molecule
13.4.3.2. Egypt
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
13.4.3.2.1. ADME
13.4.3.2.2. PK
13.4.3.2.3. PD
13.4.3.2.4. Bioavailability
13.4.3.2.5. Bioequivalence
13.4.4. Kuwait
13.4.4.1. Kuwait
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
13.4.4.1.1. Small
Molecule
13.4.4.1.2. Large
Molecule
13.4.4.2. Kuwait
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
13.4.4.2.1. ADME
13.4.4.2.2. PK
13.4.4.2.3. PD
13.4.4.2.4. Bioavailability
13.4.4.2.5. Bioequivalence
13.4.5. South
Africa
13.4.5.1. South
Africa Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
13.4.5.1.1. Small
Molecule
13.4.5.1.2. Large
Molecule
13.4.5.2. South
Africa Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Test type
13.4.5.2.1. ADME
13.4.5.2.2. PK
13.4.5.2.3. PD
13.4.5.2.4. Bioavailability
13.4.5.2.5. Bioequivalence
13.4.6. Rest of
Middle East & Africa
13.4.6.1. Rest
of Middle East & Africa Bioanalytical testing services Market Revenue (US$
Mn) and Forecasts, By Molecule type
13.4.6.1.1. Small
Molecule
13.4.6.1.2. Large
Molecule
13.4.6.2. Rest
of Middle East & Africa Bioanalytical testing services Market Revenue (US$
Mn) and Forecasts, By Test type
13.4.6.2.1. ADME
13.4.6.2.2. PK
13.4.6.2.3. PD
13.4.6.2.4. Bioavailability
13.4.6.2.5. Bioequivalence
13.5. Key
Segment for Channeling Investments
13.5.1. By
Country
13.5.2. By
Molecule type
13.5.3. By Test
type
14. Latin America Bioanalytical testing services Market Analysis
and Forecasts, 2019 - 2027
14.1. Overview
14.1.1. Latin
America Bioanalytical testing services Market Revenue (US$ Mn)
14.2. Latin
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Molecule type
14.2.1. Small
Molecule
14.2.2. Large
Molecule
14.3. Latin
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Test type
14.3.1. ADME
14.3.2. PK
14.3.3. PD
14.3.4. Bioavailability
14.3.5. Bioequivalence
14.4. Latin
America Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts,
By Country
14.4.1. Brazil
14.4.1.1. Brazil
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
14.4.1.1.1. Small
Molecule
14.4.1.1.2. Large
Molecule
14.4.1.2. Brazil
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
14.4.1.2.1. ADME
14.4.1.2.2. PK
14.4.1.2.3. PD
14.4.1.2.4. Bioavailability
14.4.1.2.5. Bioequivalence
14.4.2. Argentina
14.4.2.1. Argentina
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By
Molecule type
14.4.2.1.1. Small
Molecule
14.4.2.1.2. Large
Molecule
14.4.2.2. Argentina
Bioanalytical testing services Market Revenue (US$ Mn) and Forecasts, By Test
type
14.4.2.2.1. ADME
14.4.2.2.2. PK
14.4.2.2.3. PD
14.4.2.2.4. Bioavailability
14.4.2.2.5. Bioequivalence
14.4.3. Rest of
Latin America
14.4.3.1. Rest
of Latin America Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Molecule type
14.4.3.1.1. Small
Molecule
14.4.3.1.2. Large
Molecule
14.4.3.2. Rest
of Latin America Bioanalytical testing services Market Revenue (US$ Mn) and
Forecasts, By Test type
14.4.3.2.1. ADME
14.4.3.2.2. PK
14.4.3.2.3. PD
14.4.3.2.4. Bioavailability
14.4.3.2.5. Bioequivalence
14.5. Key
Segment for Channeling Investments
14.5.1. By
Country
14.5.2. By
Molecule type
14.5.3. By Test
type
15. Competitive Benchmarking
15.1. Market
Share Analysis, 2018
15.2. Global
Presence and Growth Strategies
15.2.1. Mergers
and Acquisitions
15.2.2. Product
Launches
15.2.3. Investments
Trends
15.2.4. R&D
Initiatives
16. Player Profiles
16.1. Bioclin
Research Laboratories
16.1.1. Company
Details
16.1.2. Company
Overview
16.1.3. Product
Offerings
16.1.4. Key
Developments
16.1.5. Financial
Analysis
16.1.6. SWOT
Analysis
16.1.7. Business
Strategies
16.2. BioReliance
Corporation
16.2.1. Company
Details
16.2.2. Company
Overview
16.2.3. Product
Offerings
16.2.4. Key
Developments
16.2.5. Financial
Analysis
16.2.6. SWOT
Analysis
16.2.7. Business
Strategies
16.3. Charles
River Laboratories International Inc.
16.3.1. Company
Details
16.3.2. Company
Overview
16.3.3. Product
Offerings
16.3.4. Key
Developments
16.3.5. Financial
Analysis
16.3.6. SWOT
Analysis
16.3.7. Business
Strategies
16.4. Eurofins
Scientific SE
16.4.1. Company
Details
16.4.2. Company
Overview
16.4.3. Product
Offerings
16.4.4. Key
Developments
16.4.5. Financial
Analysis
16.4.6. SWOT
Analysis
16.4.7. Business
Strategies
16.5. Intertek
Group Plc.
16.5.1. Company
Details
16.5.2. Company
Overview
16.5.3. Product
Offerings
16.5.4. Key
Developments
16.5.5. Financial
Analysis
16.5.6. SWOT
Analysis
16.5.7. Business
Strategies
16.6. LabCorp
16.6.1. Company
Details
16.6.2. Company
Overview
16.6.3. Product
Offerings
16.6.4. Key
Developments
16.6.5. Financial
Analysis
16.6.6. SWOT
Analysis
16.6.7. Business
Strategies
16.7. PPD Inc.
16.7.1. Company
Details
16.7.2. Company
Overview
16.7.3. Product
Offerings
16.7.4. Key
Developments
16.7.5. Financial
Analysis
16.7.6. SWOT
Analysis
16.7.7. Business
Strategies
16.8. PRA Health Sciences
16.8.1. Company
Details
16.8.2. Company
Overview
16.8.3. Product
Offerings
16.8.4. Key
Developments
16.8.5. Financial
Analysis
16.8.6. SWOT
Analysis
16.8.7. Business
Strategies
16.9. SGS
16.9.1. Company
Details
16.9.2. Company
Overview
16.9.3. Product
Offerings
16.9.4. Key
Developments
16.9.5. Financial
Analysis
16.9.6. SWOT
Analysis
16.9.7. Business
Strategies
16.10. WuXi
PharmaTech (Cayman) Inc.
16.10.1. Company
Details
16.10.2. Company
Overview
16.10.3. Product
Offerings
16.10.4. Key
Developments
16.10.5. Financial
Analysis
16.10.6. SWOT
Analysis
16.10.7. Business Strategies
16.11 Other
Market Participants
17. Key Findings
Note: This ToC is tentative
and can be changed according to the research study conducted during the course
of report completion.
**Exclusive for Multi-User and
Enterprise User.
At Absolute Markets Insights, we are engaged in building both global as well as country specific reports. As a result, the approach taken for deriving the estimation and forecast for a specific country is a bit unique and different in comparison to the global research studies. In this case, we not only study the concerned market factors & trends prevailing in a particular country (from secondary research) but we also tend to calculate the actual market size & forecast from the revenue generated from the market participants involved in manufacturing or distributing the any concerned product. These companies can also be service providers. For analyzing any country specifically, we do consider the growth factors prevailing under the states/cities/county for the same. For instance, if we are analyzing an industry specific to United States, we primarily need to study about the states present under the same(where the product/service has the highest growth). Similar analysis will be followed by other countries. Our scope of the report changes with different markets.
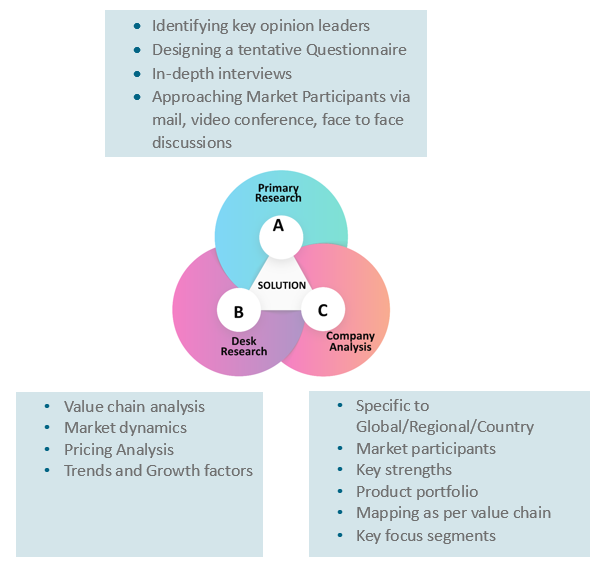
Our research study is mainly implement through a mix of both secondary and primary research. Various sources such as industry magazines, trade journals, and government websites and trade associations are reviewed for gathering precise data. Primary interviews are conducted to validate the market size derived from secondary research. Industry experts, major manufacturers and distributors are contacted for further validation purpose on the current market penetration and growth trends.
Prominent participants in our primary research process include:
- Key Opinion Leaders namely the CEOs, CSOs, VPs, purchasing managers, amongst others
- Research and development participants, distributors/suppliers and subject matter experts
Secondary Research includes data extracted from paid data sources:
- Reuters
- Factiva
- Bloomberg
- One Source
- Hoovers
Research Methodology
Key Inclusions

Why Absolute Markets Insights?
An effective strategy is the entity that influences a business to stand out of the crowd. An organization with a phenomenal strategy for success dependably has the edge over the rivals in the market. It offers the organizations a head start in planning their strategy. Absolute Market Insights is the new initiation in the industry that will furnish you with the lead your business needs. Absolute Market Insights is the best destination for your business intelligence and analytical solutions; essentially because our qualitative and quantitative sources of information are competent to give one-stop solutions. We inventively combine qualitative and quantitative research in accurate proportions to have the best report, which not only gives the most recent insights but also assists you to grow.