Electrophysiology Devices Market By Offerings (Access Device, Accessories, Cardiac Resynchronization Therapy Defibrillator, Cardiac Resynchronization Therapy Pacemakers (CRT-P), Capital Equipment: Mapping & Navigation, Capital Equipment: Therapy, Catheters, Capital Equipment: Diagnostic, Defibrillators (ICD), Leads, Left Atrial Appendage Closure, Pace Makers, Remote Patient Monitoring and Diagnostic Monitoring, Stimulators, WPI amplifiers and isolators and Others); By Clinical Indication (Aortic stenosis and mitral regurgitation, Atrial Fibrillation (AF), Supraventricular Tachycardia, Atrioventricular Nodal Re-entry Tachycardia (AVNRT), Wolff-Parkinson-White Syndrome (WPW), Bradycardia and Arrhythmias and Others); By End Users (Hospitals, Clinics, Ambulatory Care Centers, Diagnostic Centers and Others); By Region (U.S., Canada, Mexico, Rest of North America, France, UK, Germany, Spain, Italy, Nordic Countries, Benelux Union, Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia, Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of MEA, Brazil, Argentina, Rest of Latin America) – Global Insights, Growth, Size, Comparative Analysis, Trends and Forecast, 2020 – 2030
Industry Trends
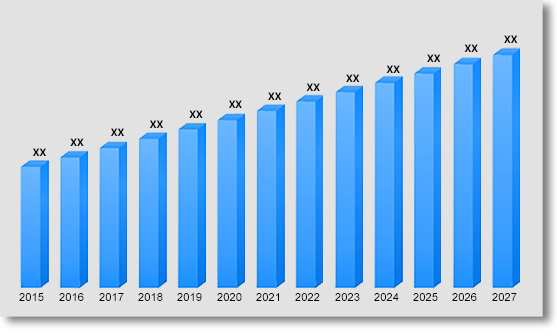
The global electrophysiology devices market was valued at US$ 5528.36 million in 2019 and is projected to grow at a CAGR of 9.4% over the forecast period. Growing prevalence of heart failure (HF) and cardiac arrest cases among the millennial attributed to lifestyle habits such as smoking and excessive alcohol consumption is the major factor propelling the market in the coming years. By 2030, the market is expected to be worth US$ 14852.35 million.
The increasing prevalence of these diseases would boost demand for EP procedures, making it a key growth factor for the electrophysiology devices industry. For example, global ablation procedure numbers are expected to increase by 13.5% per year from 973,220 in 2017 to 1,455,000 in 2022; within this group, complex ablations (AF and VT) are expected to increase by 13.5 percent per year from 440,629 in 2017 to 830,390 in 2022.
COVID-19 has had an influence on almost every industry around the world, and electrophysiology devices market is no exception. COVID-19 is creating many issues for companies all over the world. The market is hugely impacted by the Covid-19 pandemic.
The implementation of new guidelines and regulations is stifling the industry's growth. Furthermore, insufficient access to hospitals, social distancing, and population lockout have all had an effect on business development, resulting in a slowdown in patient flow and referrals. Companies continue to provide critical services despite global disturbances caused by the COVID-19 pandemic, operating under a state of emergency.
One of the major drivers for the electrophysiology devices market is the rise in the prevalence rate of cardiovascular diseases (CVD). CVD cases continue to be the leading cause of death around the world increasing the demand for quick and effective disease treatment which in turn increases the demand for electrophysiology devices. According to the American Heart Association (AHA) data released in 2019, at least 48 percent of all adults in the United States suffer from cardiovascular disease. In the United States, the prevalence of CVD, excluding high blood pressure, is 9% among adults (24.3 million in 2016). According to the Centers for Disease Control and Prevention (CDC), atrial fibrillation is the most common type of heart arrhythmia which will affect 12.1 million people in the United States by 2030. Because of the intense competition among established players, the electrophysiology market is currently highly competitive in terms of pricing. The cardiac 3D mapping systems and EP recording systems are among the most expensive of these items. The estimated list price of a cardiac 3D mapping device is between $250,000 and $800,000.
A recording device for an EP costs USD 160,000. EP procedures are typically costly due to high capital, preparation, and maintenance costs. A catheter ablation procedure, for example, can cost anywhere between USD 4,000 and USD 6,500. This, combined with poor reimbursement scenarios in many countries, renders these devices unaffordable for a significant portion of the market.
The number of surgical procedures performed in developing countries has increased steadily over the last decade, owing to an increasing target patient population, a rising number of CVD-related deaths, and increased medical tourism. Development of wearable devices is expected to offer lucrative growth opportunities for players in the global electrophysiology devices market.
Offering Insights
In offerings category, the access device segment will generate the highest revenue in the market over the forecast period. Access devices help electro physiologists and cardiac interventionists reliably access areas of the heart during ablation and mapping procedures. The companies are designing these devices to deliver high maneuverability and enhanced physician control even during complex electrophysiology (EP) procedures.
The pacemaker segment will also grow considerably over the period. One of the major factors contributing to its rapid growth is the rising occurrence of various types of arrhythmia.
Clinical indication Insights
The atrial fibrillation is the leading segment in the clinical indication category in the electrophysiology devices market. It's a common form of arrhythmia that puts the patient at risk for stroke and blood clots. In certain cases it goes undiagnosed in clinical settings with traditional screening resulting in ineffective care at the appropriate time. As a result, improved technical instruments for diagnosis are needed to control atrial fibrillation and help address the limitations imposed by traditional technologies
Regional Insights
North America region held the highest share in 2019 and is expected to continue the same trend over the forecast period. The presence of major players in the regions is boosting the growth of the market over the period. The United States is a major market for electrophysiology devices in this region, major companies in the United States are offering its product to various end users in the U.S. as well as distributing the product to other end users in various regions according to the customer requirements/needs. The need for customized devices which fits the user requirements is provoking the companies to strengthen their R&D in order to provide state of the art products to its end users. The majority of the companies are investing more on the R&D initiatives in order or strength their product portfolio.
The United States market accounted for highest share in 2019 with a total market revenue of US$ 2466.76 Million. The market will also see a huge growth over the forecast period. Canada however will see a huge growth over the forecast period with a CAGR of 11.7%, this can be attributed to the growing prevalence of Heart Failure (HF), cardiac arrest cases, and cardiac arrhythmia in this region majorly as a result of an unhealthy lifestyle, smoking, and alcohol consumption.
Owing to technological development, the European market is experiencing growth in the electrophysiological devices market. The market will see a significant growth due to presence of huge customer base as well as major companies in U.K., Italy and France. The companies in this region are focusing on expanding its user base across Europe by opening offices in other regions. Most of the major players are adopting expansion strategy in order to grasp the major share of the market.
Companies in Europe are focused more on merger and acquisitions. The market is highly competitive with companies adhering to new product development and extensive R&D investments as sustainability strategies. The adoption of these devices is more in this region due to the cost effectiveness of the product.
The European market has market revenue of US$ 1778.47 Million in 2019 and the market will see a substantial growth over the forecast period, Germany is the major market for electrophysiology devices in Europe followed by United Kingdom. Companies are pursuing an inorganic growth strategy to expand their product range and gain a foothold in the electrophysiology devices industry.
In Asia Pacific, regulatory policies are more adaptable and business-friendly. In the coming years, increased competition in developed markets would force electrophysiology system manufacturers to concentrate even more on emerging markets. Companies in the Electrophysiology (EP) instruments and equipment industry are developing new innovations aimed at enhancing EP mapping and localization technology in order to improve profit margins and revenue. Moreover, R&D in electrophysiology devices is also expected to aid in growth of the market
Electrophysiology Devices Market Revenue & Forecast, (US$ Million), 2020 – 2030
Competitive Landscape
The report provides both, qualitative and quantitative research electrophysiology devices market, as well as provides comprehensive insights and development methods adopted by the key contenders. The report also offers extensive research on the key players in this market and details on the competitiveness of these players. Key business strategies such as mergers and acquisitions (M&A), affiliations, collaborations, and contacts adopted by these major market participants are also recognized and analysed in the report. For each company, the report studies their global presence, competitors, service offerings and specification amongst others.
The major players operating in the Electrophysiology Devices Market include Abbott, ADInstruments NZ Limited, BIOTRONIK SE & Co. KG, Boston Scientific Corporation, GENERAL ELECTRIC COMPANY, Koninklijke Philips N.V., Medical Devices Business Services, Inc., Medtronic, MicroPort Scientific Corporation, Millar, Inc., Siemens Healthcare Private Limited, Transonic, Tyche MedTech, Inc., Vanguard AG, World Precision Instruments, Zeus Industrial Products, Inc. amongst others
Electrophysiology Devices Market:
- By Offerings
- Access Device
- Steerable Sheath
- Transseptal Delivery System
- Steerable Sheath
- Accessories
- Pericardiocentesis Kits
- Others
- Cardiac Resynchronization Therapy Defibrillator
- Cardiac Resynchronization Therapy Pacemakers (CRT-P)
- Capital Equipment: Diagnostic
- Ultrasound Imaging System and Ultrasound Imaging Catheter
- EP Recording System
- Capital Equipment: Mapping & Navigation
- Capital Equipment: Therapy
- Catheters
- Ablation
- Diagnostic
- Mapping
- Defibrillators (ICD)
- Leads
- Left Atrial Appendage Closure
- Pace Makers
- Remote Patient Monitoring and Diagnostic Monitoring
- Stimulators, WPI amplifiers and isolators
- Others
- Access Device
- By Clinical Indication
- Aortic stenosis and mitral regurgitation
- Atrial Fibrillation (AF)
- Supraventricular Tachycardia
- Atrioventricular Nodal Re-entry Tachycardia (AVNRT)
- Wolff-Parkinson-White Syndrome (WPW)
- Bradycardia and Arrhythmias
- Others
- By End Users
- Hospitals
- Clinics
- Ambulatory Care Centers
- Diagnostic Centers
- Others
- By Geography
- North America
- U.S
- Canada
- Mexico
- Rest of North America
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Nordic Countries
- Denmark
- Finland
- Iceland
- Sweden
- Norway
- Benelux Union
- Belgium
- The Netherlands
- Luxembourg
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Southeast Asia
- Indonesia
- Thailand
- Malaysia
- Singapore
- Rest of Southeast Asia
- Rest of Asia Pacific
- Middle East and Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- North America
Table of Contents
![]()
1 Market Scope
1.2.
Market Segmentation
1.3.
Years Considered
1.3.12.
Historic Years: 2015 - 2018
1.3.13.
Base Year: 2019
1.3.14.
Forecast Years: 2020 – 2030
2 Key Target Audiences
3 Research Methodology
3.2.
Primary Research
3.2.12.
Research Questionnaire
3.2.13.
Global Percentage Breakdown
3.2.14.
Primary Interviews: Key Opinion Leaders
(KOLs)
3.3.
Secondary Research
3.3.12.
Paid Databases
3.3.13.
Secondary Sources
3.4.
Market Size Estimates
3.4.12.
Top-Down Approach
3.4.13.
Bottom-Up Approach
3.5.
Data Triangulation Methodology
3.6.
Research Assumptions
4 Recommendations and Insights from AMI’s Perspective**
5 Holistic Overview of Electrophysiology (EP) Devices Market
6 Market Synopsis:
Electrophysiology (EP) Devices Market
7 Electrophysiology (EP) Devices Market Analysis: Qualitative
Perspective
7.2.
Introduction
7.2.12.
Product Definition
7.2.13.
Industry Development
7.3.
Market Dynamics
7.3.12.
Drivers
7.3.13.
Restraints
7.3.14.
Opportunities
7.3.15.
Challenges
7.4.
Trends in Electrophysiology (EP)
Devices Market
7.5.
Market Determinants Radar Chart
7.6.
Macro-Economic and Micro-Economic
Indicators: Electrophysiology (EP) Devices Market
7.7.
Industry Gross Margin Overview
7.8. Porter’s
Five Force Analysis
7.9.
Impact of Covid-19 on this market
8 Global Electrophysiology (EP) Devices Market Analysis and
Forecasts, 2020 – 2030
8.2.
Overview
8.2.12.
Global Electrophysiology (EP) Devices
Market Revenue (US$ Mn)
8.3.
Global Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
8.3.12.
Access Device (Definition, Market
Estimation and Penetration, 2015-2019, Market Estimation (2015-2019), Market
Forecast (2020 – 2030), Compound Annual Growth Rate (CAGR), Regional
Bifurcation (North America, Europe, Asia Pacific, Middle East and Africa, Latin
America) and Information on Steerable Sheath, Transseptal Delivery System)
8.3.12.1.
Steerable Sheath
8.3.12.2.
Transseptal Delivery System
8.3.13.
Accessories (Definition, Market
Estimation and Penetration, 2015-2019, Market Estimation (2015-2019), Market
Forecast (2020 – 2030), Compound Annual Growth Rate (CAGR), Regional
Bifurcation (North America, Europe, Asia Pacific, Middle East and Africa, Latin
America) and Information on Pericardiocentesis Kits, Others)
8.3.13.1.
Pericardiocentesis Kits
8.3.13.2.
Others
8.3.14.
Cardiac Resynchronization Therapy
Defibrillator
8.3.14.1.
Definition
8.3.14.2.
Market Estimation and Penetration,
2015-2019
8.3.14.3.
Market Forecast, 2020 – 2030
8.3.14.4.
Compound Annual Growth Rate (CAGR)
8.3.14.5.
Regional Bifurcation
8.3.14.5.1.
North America
8.3.14.5.1.1.
Market Estimation, 2015-2019
8.3.14.5.1.2.
Market Forecast, 2020 – 2030
8.3.14.5.2.
Europe
8.3.14.5.2.1.
Market Estimation, 2015-2019
8.3.14.5.2.2.
Market Forecast, 2020 – 2030
8.3.14.5.3.
Asia Pacific
8.3.14.5.3.1.
Market Estimation, 2015-2019
8.3.14.5.3.2.
Market Forecast, 2020 – 2030
8.3.14.5.4.
Middle East and Africa
8.3.14.5.4.1.
Market Estimation, 2015-2019
8.3.14.5.4.2.
Market Forecast, 2020 – 2030
8.3.14.5.5.
Latin America
8.3.14.5.5.1.
Market Estimation, 2015-2019
8.3.14.5.5.2.
Market Forecast, 2020 – 2030
8.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
8.3.15.1.
Definition
8.3.15.2.
Market Estimation and Penetration,
2015-2019
8.3.15.3.
Market Forecast, 2020 – 2030
8.3.15.4.
Compound Annual Growth Rate (CAGR)
8.3.15.5.
Regional Bifurcation
8.3.15.5.1.
North America
8.3.15.5.1.1.
Market Estimation, 2015-2019
8.3.15.5.1.2.
Market Forecast, 2020 – 2030
8.3.15.5.2.
Europe
8.3.15.5.2.1.
Market Estimation, 2015-2019
8.3.15.5.2.2.
Market Forecast, 2020 – 2030
8.3.15.5.3.
Asia Pacific
8.3.15.5.3.1.
Market Estimation, 2015-2019
8.3.15.5.3.2.
Market Forecast, 2020 – 2030
8.3.15.5.4.
Middle East and Africa
8.3.15.5.4.1.
Market Estimation, 2015-2019
8.3.15.5.4.2.
Market Forecast, 2020 – 2030
8.3.15.5.5.
Latin America
8.3.15.5.5.1.
Market Estimation, 2015-2019
8.3.15.5.5.2.
Market Forecast, 2020 – 2030
8.3.16.
Capital Equipment: Diagnostic
(Definition, Market Estimation and Penetration, 2015-2019, Market Estimation
(2015-2019), Market Forecast (2020 – 2030), Compound Annual Growth Rate (CAGR),
Regional Bifurcation (North America, Europe, Asia Pacific, Middle East and
Africa, Latin America) and Information on Ultrasound Imaging System and
Ultrasound Imaging Catheter, EP Recording System)
8.3.16.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
8.3.16.2.
EP Recording System
8.3.17.
Capital Equipment: Mapping and
Navigation
8.3.17.1.
Definition
8.3.17.2.
Market Estimation and Penetration,
2015-2019
8.3.17.3.
Market Forecast, 2020 – 2030
8.3.17.4.
Compound Annual Growth Rate (CAGR)
8.3.17.5.
Regional Bifurcation
8.3.17.5.1.
North America
8.3.17.5.1.1.
Market Estimation, 2015-2019
8.3.17.5.1.2.
Market Forecast, 2020 – 2030
8.3.17.5.2.
Europe
8.3.17.5.2.1.
Market Estimation, 2015-2019
8.3.17.5.2.2.
Market Forecast, 2020 – 2030
8.3.17.5.3.
Asia Pacific
8.3.17.5.3.1.
Market Estimation, 2015-2019
8.3.17.5.3.2.
Market Forecast, 2020 – 2030
8.3.17.5.4.
Middle East and Africa
8.3.17.5.4.1.
Market Estimation, 2015-2019
8.3.17.5.4.2.
Market Forecast, 2020 – 2030
8.3.17.5.5.
Latin America
8.3.17.5.5.1.
Market Estimation, 2015-2019
8.3.17.5.5.2.
Market Forecast, 2020 – 2030
8.3.18.
Capital Equipment: Therapy
8.3.18.1.
Definition
8.3.18.2.
Market Estimation and Penetration,
2015-2019
8.3.18.3.
Market Forecast, 2020 – 2030
8.3.18.4.
Compound Annual Growth Rate (CAGR)
8.3.18.5.
Regional Bifurcation
8.3.18.5.1.
North America
8.3.18.5.1.1.
Market Estimation, 2015-2019
8.3.18.5.1.2.
Market Forecast, 2020 – 2030
8.3.18.5.2.
Europe
8.3.18.5.2.1.
Market Estimation, 2015-2019
8.3.18.5.2.2.
Market Forecast, 2020 – 2030
8.3.18.5.3.
Asia Pacific
8.3.18.5.3.1.
Market Estimation, 2015-2019
8.3.18.5.3.2.
Market Forecast, 2020 – 2030
8.3.18.5.4.
Middle East and Africa
8.3.18.5.4.1.
Market Estimation, 2015-2019
8.3.18.5.4.2.
Market Forecast, 2020 – 2030
8.3.18.5.5.
Latin America
8.3.18.5.5.1.
Market Estimation, 2015-2019
8.3.18.5.5.2.
Market Forecast, 2020 – 2030
8.3.19.
Catheters (Definition, Market
Estimation and Penetration, 2015-2019, Market Estimation (2015-2019), Market
Forecast (2020 – 2030), Compound Annual Growth Rate (CAGR), Regional
Bifurcation (North America, Europe, Asia Pacific, Middle East and Africa, Latin
America) and Information on Ablation, Diagnostic, Mapping)
8.3.19.1.
Ablation
8.3.19.2.
Diagnostic
8.3.19.3.
Mapping
8.3.20.
Defibrillators (ICD)
8.3.20.1.
Definition
8.3.20.2.
Market Estimation and Penetration,
2015-2019
8.3.20.3.
Market Forecast, 2020 – 2030
8.3.20.4.
Compound Annual Growth Rate (CAGR)
8.3.20.5.
Regional Bifurcation
8.3.20.5.1.
North America
8.3.20.5.1.1.
Market Estimation, 2015-2019
8.3.20.5.1.2.
Market Forecast, 2020 – 2030
8.3.20.5.2.
Europe
8.3.20.5.2.1.
Market Estimation, 2015-2019
8.3.20.5.2.2.
Market Forecast, 2020 – 2030
8.3.20.5.3.
Asia Pacific
8.3.20.5.3.1.
Market Estimation, 2015-2019
8.3.20.5.3.2.
Market Forecast, 2020 – 2030
8.3.20.5.4.
Middle East and Africa
8.3.20.5.4.1.
Market Estimation, 2015-2019
8.3.20.5.4.2.
Market Forecast, 2020 – 2030
8.3.20.5.5.
Latin America
8.3.20.5.5.1.
Market Estimation, 2015-2019
8.3.20.5.5.2.
Market Forecast, 2020 – 2030
8.3.21.
Leads
8.3.21.1.
Definition
8.3.21.2.
Market Estimation and Penetration,
2015-2019
8.3.21.3.
Market Forecast, 2020 – 2030
8.3.21.4.
Compound Annual Growth Rate (CAGR)
8.3.21.5.
Regional Bifurcation
8.3.21.5.1.
North America
8.3.21.5.1.1.
Market Estimation, 2015-2019
8.3.21.5.1.2.
Market Forecast, 2020 – 2030
8.3.21.5.2.
Europe
8.3.21.5.2.1.
Market Estimation, 2015-2019
8.3.21.5.2.2.
Market Forecast, 2020 – 2030
8.3.21.5.3.
Asia Pacific
8.3.21.5.3.1.
Market Estimation, 2015-2019
8.3.21.5.3.2.
Market Forecast, 2020 – 2030
8.3.21.5.4.
Middle East and Africa
8.3.21.5.4.1.
Market Estimation, 2015-2019
8.3.21.5.4.2.
Market Forecast, 2020 – 2030
8.3.21.5.5.
Latin America
8.3.21.5.5.1.
Market Estimation, 2015-2019
8.3.21.5.5.2.
Market Forecast, 2020 – 2030
8.3.22.
Left Atrial Appendage Closure
8.3.22.1.
Definition
8.3.22.2.
Market Estimation and Penetration,
2015-2019
8.3.22.3.
Market Forecast, 2020 – 2030
8.3.22.4.
Compound Annual Growth Rate (CAGR)
8.3.22.5.
Regional Bifurcation
8.3.22.5.1.
North America
8.3.22.5.1.1.
Market Estimation, 2015-2019
8.3.22.5.1.2.
Market Forecast, 2020 – 2030
8.3.22.5.2.
Europe
8.3.22.5.2.1.
Market Estimation, 2015-2019
8.3.22.5.2.2.
Market Forecast, 2020 – 2030
8.3.22.5.3.
Asia Pacific
8.3.22.5.3.1.
Market Estimation, 2015-2019
8.3.22.5.3.2.
Market Forecast, 2020 – 2030
8.3.22.5.4.
Middle East and Africa
8.3.22.5.4.1.
Market Estimation, 2015-2019
8.3.22.5.4.2.
Market Forecast, 2020 – 2030
8.3.22.5.5.
Latin America
8.3.22.5.5.1.
Market Estimation, 2015-2019
8.3.22.5.5.2.
Market Forecast, 2020 – 2030
8.3.23.
Pace Makers
8.3.23.1.
Definition
8.3.23.2.
Market Estimation and Penetration,
2015-2019
8.3.23.3.
Market Forecast, 2020 – 2030
8.3.23.4.
Compound Annual Growth Rate (CAGR)
8.3.23.5.
Regional Bifurcation
8.3.23.5.1.
North America
8.3.23.5.1.1.
Market Estimation, 2015-2019
8.3.23.5.1.2.
Market Forecast, 2020 – 2030
8.3.23.5.2.
Europe
8.3.23.5.2.1.
Market Estimation, 2015-2019
8.3.23.5.2.2.
Market Forecast, 2020 – 2030
8.3.23.5.3.
Asia Pacific
8.3.23.5.3.1.
Market Estimation, 2015-2019
8.3.23.5.3.2.
Market Forecast, 2020 – 2030
8.3.23.5.4.
Middle East and Africa
8.3.23.5.4.1.
Market Estimation, 2015-2019
8.3.23.5.4.2.
Market Forecast, 2020 – 2030
8.3.23.5.5.
Latin America
8.3.23.5.5.1.
Market Estimation, 2015-2019
8.3.23.5.5.2.
Market Forecast, 2020 – 2030
8.3.24.
Remote Patient Monitoring and
Diagnostic Monitoring
8.3.24.1.
Definition
8.3.24.2.
Market Estimation and Penetration, 2015-2019
8.3.24.3.
Market Forecast, 2020 – 2030
8.3.24.4.
Compound Annual Growth Rate (CAGR)
8.3.24.5.
Regional Bifurcation
8.3.24.5.1.
North America
8.3.24.5.1.1.
Market Estimation, 2015-2019
8.3.24.5.1.2.
Market Forecast, 2020 – 2030
8.3.24.5.2.
Europe
8.3.24.5.2.1.
Market Estimation, 2015-2019
8.3.24.5.2.2.
Market Forecast, 2020 – 2030
8.3.24.5.3.
Asia Pacific
8.3.24.5.3.1.
Market Estimation, 2015-2019
8.3.24.5.3.2.
Market Forecast, 2020 – 2030
8.3.24.5.4.
Middle East and Africa
8.3.24.5.4.1.
Market Estimation, 2015-2019
8.3.24.5.4.2.
Market Forecast, 2020 – 2030
8.3.24.5.5.
Latin America
8.3.24.5.5.1.
Market Estimation, 2015-2019
8.3.24.5.5.2.
Market Forecast, 2020 – 2030
8.3.25.
Stimulators, WPI amplifiers and isolators
8.3.25.1.
Definition
8.3.25.2.
Market Estimation and Penetration,
2015-2019
8.3.25.3.
Market Forecast, 2020 – 2030
8.3.25.4.
Compound Annual Growth Rate (CAGR)
8.3.25.5.
Regional Bifurcation
8.3.25.5.1.
North America
8.3.25.5.1.1.
Market Estimation, 2015-2019
8.3.25.5.1.2.
Market Forecast, 2020 – 2030
8.3.25.5.2.
Europe
8.3.25.5.2.1.
Market Estimation, 2015-2019
8.3.25.5.2.2.
Market Forecast, 2020 – 2030
8.3.25.5.3.
Asia Pacific
8.3.25.5.3.1.
Market Estimation, 2015-2019
8.3.25.5.3.2.
Market Forecast, 2020 – 2030
8.3.25.5.4.
Middle East and Africa
8.3.25.5.4.1.
Market Estimation, 2015-2019
8.3.25.5.4.2.
Market Forecast, 2020 – 2030
8.3.25.5.5.
Latin America
8.3.25.5.5.1.
Market Estimation, 2015-2019
8.3.25.5.5.2.
Market Forecast, 2020 – 2030
8.3.26.
Others
8.3.26.1.
Definition
8.3.26.2.
Market Estimation and Penetration,
2015-2019
8.3.26.3.
Market Forecast, 2020 – 2030
8.3.26.4.
Compound Annual Growth Rate (CAGR)
8.3.26.5.
Regional Bifurcation
8.3.26.5.1.
North America
8.3.26.5.1.1.
Market Estimation, 2015-2019
8.3.26.5.1.2.
Market Forecast, 2020 – 2030
8.3.26.5.2.
Europe
8.3.26.5.2.1.
Market Estimation, 2015-2019
8.3.26.5.2.2.
Market Forecast, 2020 – 2030
8.3.26.5.3.
Asia Pacific
8.3.26.5.3.1.
Market Estimation, 2015-2019
8.3.26.5.3.2.
Market Forecast, 2020 – 2030
8.3.26.5.4.
Middle East and Africa
8.3.26.5.4.1.
Market Estimation, 2015-2019
8.3.26.5.4.2.
Market Forecast, 2020 – 2030
8.3.26.5.5.
Latin America
8.3.26.5.5.1.
Market Estimation, 2015-2019
8.3.26.5.5.2.
Market Forecast, 2020 – 2030
8.4.
Key Segment for Channeling Investments
8.4.12.
By Offerings
9 Global Electrophysiology (EP) Devices Market Analysis and
Forecasts, 2020 – 2030
9.2.
Overview
9.3.
Global Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
9.3.12.
Aortic stenosis and mitral
regurgitation
9.3.12.1.
Definition
9.3.12.2.
Market Estimation and Penetration,
2015-2019
9.3.12.3.
Market Forecast, 2020 – 2030
9.3.12.4.
Compound Annual Growth Rate (CAGR)
9.3.12.5.
Regional Bifurcation
9.3.12.5.1.
North America
9.3.12.5.1.1.
Market Estimation, 2015-2019
9.3.12.5.1.2.
Market Forecast, 2020 – 2030
9.3.12.5.2.
Europe
9.3.12.5.2.1.
Market Estimation, 2015-2019
9.3.12.5.2.2.
Market Forecast, 2020 – 2030
9.3.12.5.3.
Asia Pacific
9.3.12.5.3.1.
Market Estimation, 2015-2019
9.3.12.5.3.2.
Market Forecast, 2020 – 2030
9.3.12.5.4.
Middle East and Africa
9.3.12.5.4.1.
Market Estimation, 2015-2019
9.3.12.5.4.2.
Market Forecast, 2020 – 2030
9.3.12.5.5.
Latin America
9.3.12.5.5.1.
Market Estimation, 2015-2019
9.3.12.5.5.2.
Market Forecast, 2020 – 2030
9.3.13.
Atrial Fibrillation (AF)
9.3.13.1.
Definition
9.3.13.2.
Market Estimation and Penetration,
2015-2019
9.3.13.3.
Market Forecast, 2020 – 2030
9.3.13.4.
Compound Annual Growth Rate (CAGR)
9.3.13.5.
Regional Bifurcation
9.3.13.5.1.
North America
9.3.13.5.1.1.
Market Estimation, 2015-2019
9.3.13.5.1.2.
Market Forecast, 2020 – 2030
9.3.13.5.2.
Europe
9.3.13.5.2.1.
Market Estimation, 2015-2019
9.3.13.5.2.2.
Market Forecast, 2020 – 2030
9.3.13.5.3.
Asia Pacific
9.3.13.5.3.1.
Market Estimation, 2015-2019
9.3.13.5.3.2.
Market Forecast, 2020 – 2030
9.3.13.5.4.
Middle East and Africa
9.3.13.5.4.1.
Market Estimation, 2015-2019
9.3.13.5.4.2.
Market Forecast, 2020 – 2030
9.3.13.5.5.
Latin America
9.3.13.5.5.1.
Market Estimation, 2015-2019
9.3.13.5.5.2.
Market Forecast, 2020 – 2030
9.3.14.
Supraventricular Tachycardia
9.3.14.1.
Definition
9.3.14.2.
Market Estimation and Penetration,
2015-2019
9.3.14.3.
Market Forecast, 2020 – 2030
9.3.14.4.
Compound Annual Growth Rate (CAGR)
9.3.14.5.
Regional Bifurcation
9.3.14.5.1.
North America
9.3.14.5.1.1.
Market Estimation, 2015-2019
9.3.14.5.1.2.
Market Forecast, 2020 – 2030
9.3.14.5.2.
Europe
9.3.14.5.2.1.
Market Estimation, 2015-2019
9.3.14.5.2.2.
Market Forecast, 2020 – 2030
9.3.14.5.3.
Asia Pacific
9.3.14.5.3.1.
Market Estimation, 2015-2019
9.3.14.5.3.2.
Market Forecast, 2020 – 2030
9.3.14.5.4.
Middle East and Africa
9.3.14.5.4.1.
Market Estimation, 2015-2019
9.3.14.5.4.2.
Market Forecast, 2020 – 2030
9.3.14.5.5.
Latin America
9.3.14.5.5.1.
Market Estimation, 2015-2019
9.3.14.5.5.2.
Market Forecast, 2020 – 2030
9.3.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
9.3.15.1.
Definition
9.3.15.2.
Market Estimation and Penetration,
2015-2019
9.3.15.3.
Market Forecast, 2020 – 2030
9.3.15.4.
Compound Annual Growth Rate (CAGR)
9.3.15.5.
Regional Bifurcation
9.3.15.5.1.
North America
9.3.15.5.1.1.
Market Estimation, 2015-2019
9.3.15.5.1.2.
Market Forecast, 2020 – 2030
9.3.15.5.2.
Europe
9.3.15.5.2.1.
Market Estimation, 2015-2019
9.3.15.5.2.2.
Market Forecast, 2020 – 2030
9.3.15.5.3.
Asia Pacific
9.3.15.5.3.1.
Market Estimation, 2015-2019
9.3.15.5.3.2.
Market Forecast, 2020 – 2030
9.3.15.5.4.
Middle East and Africa
9.3.15.5.4.1.
Market Estimation, 2015-2019
9.3.15.5.4.2.
Market Forecast, 2020 – 2030
9.3.15.5.5.
Latin America
9.3.15.5.5.1.
Market Estimation, 2015-2019
9.3.15.5.5.2.
Market Forecast, 2020 – 2030
9.3.16.
Wolff-Parkinson-White Syndrome (WPW)
9.3.16.1.
Definition
9.3.16.2.
Market Estimation and Penetration,
2015-2019
9.3.16.3.
Market Forecast, 2020 – 2030
9.3.16.4.
Compound Annual Growth Rate (CAGR)
9.3.16.5.
Regional Bifurcation
9.3.16.5.1.
North America
9.3.16.5.1.1.
Market Estimation, 2015-2019
9.3.16.5.1.2.
Market Forecast, 2020 – 2030
9.3.16.5.2.
Europe
9.3.16.5.2.1.
Market Estimation, 2015-2019
9.3.16.5.2.2.
Market Forecast, 2020 – 2030
9.3.16.5.3.
Asia Pacific
9.3.16.5.3.1.
Market Estimation, 2015-2019
9.3.16.5.3.2.
Market Forecast, 2020 – 2030
9.3.16.5.4.
Middle East and Africa
9.3.16.5.4.1.
Market Estimation, 2015-2019
9.3.16.5.4.2.
Market Forecast, 2020 – 2030
9.3.16.5.5.
Latin America
9.3.16.5.5.1.
Market Estimation, 2015-2019
9.3.16.5.5.2.
Market Forecast, 2020 – 2030
9.3.17.
Bradycardia and Arrhythmias
9.3.17.1.
Definition
9.3.17.2.
Market Estimation and Penetration,
2015-2019
9.3.17.3.
Market Forecast, 2020 – 2030
9.3.17.4.
Compound Annual Growth Rate (CAGR)
9.3.17.5.
Regional Bifurcation
9.3.17.5.1.
North America
9.3.17.5.1.1.
Market Estimation, 2015-2019
9.3.17.5.1.2.
Market Forecast, 2020 – 2030
9.3.17.5.2.
Europe
9.3.17.5.2.1.
Market Estimation, 2015-2019
9.3.17.5.2.2.
Market Forecast, 2020 – 2030
9.3.17.5.3.
Asia Pacific
9.3.17.5.3.1.
Market Estimation, 2015-2019
9.3.17.5.3.2.
Market Forecast, 2020 – 2030
9.3.17.5.4.
Middle East and Africa
9.3.17.5.4.1.
Market Estimation, 2015-2019
9.3.17.5.4.2.
Market Forecast, 2020 – 2030
9.3.17.5.5.
Latin America
9.3.17.5.5.1.
Market Estimation, 2015-2019
9.3.17.5.5.2.
Market Forecast, 2020 – 2030
9.3.18.
Others
9.3.18.1.
Definition
9.3.18.2.
Market Estimation and Penetration,
2015-2019
9.3.18.3.
Market Forecast, 2020 – 2030
9.3.18.4.
Compound Annual Growth Rate (CAGR)
9.3.18.5.
Regional Bifurcation
9.3.18.5.1.
North America
9.3.18.5.1.1.
Market Estimation, 2015-2019
9.3.18.5.1.2.
Market Forecast, 2020 – 2030
9.3.18.5.2.
Europe
9.3.18.5.2.1.
Market Estimation, 2015-2019
9.3.18.5.2.2.
Market Forecast, 2020 – 2030
9.3.18.5.3.
Asia Pacific
9.3.18.5.3.1.
Market Estimation, 2015-2019
9.3.18.5.3.2.
Market Forecast, 2020 – 2030
9.3.18.5.4.
Middle East and Africa
9.3.18.5.4.1.
Market Estimation, 2015-2019
9.3.18.5.4.2.
Market Forecast, 2020 – 2030
9.3.18.5.5.
Latin America
9.3.18.5.5.1.
Market Estimation, 2015-2019
9.3.18.5.5.2.
Market Forecast, 2020 – 2030
9.4.
Key Segment for Channeling Investments
9.4.12.
By Clinical Indication
10 Global Electrophysiology (EP) Devices Market Analysis and
Forecasts, 2020 – 2030
10.2.
Overview
10.3.
Global Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
10.3.12.
Hospitals
10.3.12.1.
Definition
10.3.12.2.
Market Estimation and Penetration,
2015-2019
10.3.12.3.
Market Forecast, 2020 – 2030
10.3.12.4.
Compound Annual Growth Rate (CAGR)
10.3.12.5.
Regional Bifurcation
10.3.12.5.1.
North America
10.3.12.5.1.1.
Market Estimation, 2015-2019
10.3.12.5.1.2.
Market Forecast, 2020 – 2030
10.3.12.5.2.
Europe
10.3.12.5.2.1.
Market Estimation, 2015-2019
10.3.12.5.2.2.
Market Forecast, 2020 – 2030
10.3.12.5.3.
Asia Pacific
10.3.12.5.3.1.
Market Estimation, 2015-2019
10.3.12.5.3.2.
Market Forecast, 2020 – 2030
10.3.12.5.4.
Middle East and Africa
10.3.12.5.4.1.
Market Estimation, 2015-2019
10.3.12.5.4.2.
Market Forecast, 2020 – 2030
10.3.12.5.5.
Latin America
10.3.12.5.5.1.
Market Estimation, 2015-2019
10.3.12.5.5.2.
Market Forecast, 2020 – 2030
10.3.13.
Clinics
10.3.13.1.
Definition
10.3.13.2.
Market Estimation and Penetration,
2015-2019
10.3.13.3.
Market Forecast, 2020 – 2030
10.3.13.4.
Compound Annual Growth Rate (CAGR)
10.3.13.5.
Regional Bifurcation
10.3.13.5.1.
North America
10.3.13.5.1.1.
Market Estimation, 2015-2019
10.3.13.5.1.2.
Market Forecast, 2020 – 2030
10.3.13.5.2.
Europe
10.3.13.5.2.1.
Market Estimation, 2015-2019
10.3.13.5.2.2.
Market Forecast, 2020 – 2030
10.3.13.5.3.
Asia Pacific
10.3.13.5.3.1.
Market Estimation, 2015-2019
10.3.13.5.3.2.
Market Forecast, 2020 – 2030
10.3.13.5.4.
Middle East and Africa
10.3.13.5.4.1.
Market Estimation, 2015-2019
10.3.13.5.4.2.
Market Forecast, 2020 – 2030
10.3.13.5.5.
Latin America
10.3.13.5.5.1.
Market Estimation, 2015-2019
10.3.13.5.5.2.
Market Forecast, 2020 – 2030
10.3.14.
Ambulatory Care Centers
10.3.14.1.
Definition
10.3.14.2.
Market Estimation and Penetration,
2015-2019
10.3.14.3.
Market Forecast, 2020 – 2030
10.3.14.4.
Compound Annual Growth Rate (CAGR)
10.3.14.5.
Regional Bifurcation
10.3.14.5.1.
North America
10.3.14.5.1.1.
Market Estimation, 2015-2019
10.3.14.5.1.2.
Market Forecast, 2020 – 2030
10.3.14.5.2.
Europe
10.3.14.5.2.1.
Market Estimation, 2015-2019
10.3.14.5.2.2.
Market Forecast, 2020 – 2030
10.3.14.5.3.
Asia Pacific
10.3.14.5.3.1.
Market Estimation, 2015-2019
10.3.14.5.3.2.
Market Forecast, 2020 – 2030
10.3.14.5.4.
Middle East and Africa
10.3.14.5.4.1.
Market Estimation, 2015-2019
10.3.14.5.4.2.
Market Forecast, 2020 – 2030
10.3.14.5.5.
Latin America
10.3.14.5.5.1.
Market Estimation, 2015-2019
10.3.14.5.5.2.
Market Forecast, 2020 – 2030
10.3.15.
Diagnostic Centers
10.3.15.1.
Definition
10.3.15.2.
Market Estimation and Penetration,
2015-2019
10.3.15.3.
Market Forecast, 2020 – 2030
10.3.15.4.
Compound Annual Growth Rate (CAGR)
10.3.15.5.
Regional Bifurcation
10.3.15.5.1.
North America
10.3.15.5.1.1.
Market Estimation, 2015-2019
10.3.15.5.1.2.
Market Forecast, 2020 – 2030
10.3.15.5.2.
Europe
10.3.15.5.2.1.
Market Estimation, 2015-2019
10.3.15.5.2.2.
Market Forecast, 2020 – 2030
10.3.15.5.3.
Asia Pacific
10.3.15.5.3.1.
Market Estimation, 2015-2019
10.3.15.5.3.2.
Market Forecast, 2020 – 2030
10.3.15.5.4.
Middle East and Africa
10.3.15.5.4.1.
Market Estimation, 2015-2019
10.3.15.5.4.2.
Market Forecast, 2020 – 2030
10.3.15.5.5.
Latin America
10.3.15.5.5.1.
Market Estimation, 2015-2019
10.3.15.5.5.2.
Market Forecast, 2020 – 2030
10.3.16.
Others
10.3.16.1.
Definition
10.3.16.2.
Market Estimation and Penetration,
2015-2019
10.3.16.3.
Market Forecast, 2020 – 2030
10.3.16.4.
Compound Annual Growth Rate (CAGR)
10.3.16.5.
Regional Bifurcation
10.3.16.5.1.
North America
10.3.16.5.1.1.
Market Estimation, 2015-2019
10.3.16.5.1.2.
Market Forecast, 2020 – 2030
10.3.16.5.2.
Europe
10.3.16.5.2.1.
Market Estimation, 2015-2019
10.3.16.5.2.2.
Market Forecast, 2020 – 2030
10.3.16.5.3.
Asia Pacific
10.3.16.5.3.1.
Market Estimation, 2015-2019
10.3.16.5.3.2.
Market Forecast, 2020 – 2030
10.3.16.5.4.
Middle East and Africa
10.3.16.5.4.1.
Market Estimation, 2015-2019
10.3.16.5.4.2.
Market Forecast, 2020 – 2030
10.3.16.5.5.
Latin America
10.3.16.5.5.1.
Market Estimation, 2015-2019
10.3.16.5.5.2.
Market Forecast, 2020 – 2030
10.4.
Key Segment for Channeling Investments
10.4.12.
By End-User
11 North America Electrophysiology (EP) Devices Market Analysis
and Forecasts, 2020 – 2030
11.2.
Overview
11.2.12.
North America Electrophysiology (EP)
Devices Market Revenue (US$ Mn)
11.3.
North America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
11.3.12.
Access Device
11.3.12.1.
Steerable Sheath
11.3.12.2.
Transseptal Delivery System
11.3.13.
Accessories
11.3.13.1.
Pericardiocentesis Kits
11.3.13.2.
Others
11.3.14.
Cardiac Resynchronization Therapy
Defibrillator
11.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
11.3.16.
Capital Equipment: Diagnostic
11.3.16.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
11.3.16.2.
EP Recording System
11.3.17.
Capital Equipment: Mapping and
Navigation
11.3.18.
Capital Equipment: Therapy
11.3.19.
Catheters
11.3.19.1.
Ablation
11.3.19.2.
Diagnostic
11.3.19.3.
Mapping
11.3.20.
Defibrillators (ICD)
11.3.21.
Leads
11.3.22.
Left Atrial Appendage Closure
11.3.23.
Pace Makers
11.3.24.
Remote Patient Monitoring and
Diagnostic Monitoring
11.3.25.
Stimulators, WPI amplifiers and
isolators
11.3.26.
Others
11.4.
North America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
11.4.12.
Aortic stenosis and mitral
regurgitation
11.4.13.
Atrial Fibrillation (AF)
11.4.14.
Supraventricular Tachycardia
11.4.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
11.4.16.
Wolff-Parkinson-White Syndrome (WPW)
11.4.17.
Bradycardia and Arrhythmias
11.4.18.
Others
11.5.
North America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
11.5.12.
Hospitals
11.5.13.
Clinics
11.5.14.
Ambulatory Care Centers
11.5.15.
Diagnostic Centers
11.5.16.
Others
11.6.
North America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
11.6.12.
U.S
11.6.12.1.
U.S Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
11.6.12.1.1.
Access Device
11.6.12.1.1.1.
Steerable Sheath
11.6.12.1.1.2.
Transseptal Delivery System
11.6.12.1.2.
Accessories
11.6.12.1.2.1.
Pericardiocentesis Kits
11.6.12.1.2.2.
Others
11.6.12.1.3.
Cardiac Resynchronization Therapy
Defibrillator
11.6.12.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
11.6.12.1.5.
Capital Equipment: Diagnostic
11.6.12.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
11.6.12.1.5.2.
EP Recording System
11.6.12.1.6.
Capital Equipment: Mapping and
Navigation
11.6.12.1.7.
Capital Equipment: Therapy
11.6.12.1.8.
Catheters
11.6.12.1.8.1.
Ablation
11.6.12.1.8.2.
Diagnostic
11.6.12.1.8.3.
Mapping
11.6.12.1.9.
Defibrillators (ICD)
11.6.12.1.10.
Leads
11.6.12.1.11.
Left Atrial Appendage Closure
11.6.12.1.12.
Pace Makers
11.6.12.1.13.
Remote Patient Monitoring and Diagnostic
Monitoring
11.6.12.1.14.
Stimulators, WPI amplifiers and
isolators
11.6.12.1.15.
Others
11.6.12.2.
U.S Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
11.6.12.2.1.
Aortic stenosis and mitral
regurgitation
11.6.12.2.2.
Atrial Fibrillation (AF)
11.6.12.2.3.
Supraventricular Tachycardia
11.6.12.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
11.6.12.2.5.
Wolff-Parkinson-White Syndrome (WPW)
11.6.12.2.6.
Bradycardia and Arrhythmias
11.6.12.2.7.
Others
11.6.12.3.
U.S Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
11.6.12.3.1.
Hospitals
11.6.12.3.2.
Clinics
11.6.12.3.3.
Ambulatory Care Centers
11.6.12.3.4.
Diagnostic Centers
11.6.12.3.5.
Others
11.6.13.
Canada
11.6.13.1.
Canada Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
11.6.13.1.1.
Access Device
11.6.13.1.1.1.
Steerable Sheath
11.6.13.1.1.2.
Transseptal Delivery System
11.6.13.1.2.
Accessories
11.6.13.1.2.1.
Pericardiocentesis Kits
11.6.13.1.2.2.
Others
11.6.13.1.3.
Cardiac Resynchronization Therapy
Defibrillator
11.6.13.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
11.6.13.1.5.
Capital Equipment: Diagnostic
11.6.13.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
11.6.13.1.5.2.
EP Recording System
11.6.13.1.6.
Capital Equipment: Mapping and
Navigation
11.6.13.1.7.
Capital Equipment: Therapy
11.6.13.1.8.
Catheters
11.6.13.1.8.1.
Ablation
11.6.13.1.8.2.
Diagnostic
11.6.13.1.8.3.
Mapping
11.6.13.1.9.
Defibrillators (ICD)
11.6.13.1.10.
Leads
11.6.13.1.11.
Left Atrial Appendage Closure
11.6.13.1.12.
Pace Makers
11.6.13.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
11.6.13.1.14.
Stimulators, WPI amplifiers and
isolators
11.6.13.1.15.
Others
11.6.13.2.
Canada Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
11.6.13.2.1.
Aortic stenosis and mitral
regurgitation
11.6.13.2.2.
Atrial Fibrillation (AF)
11.6.13.2.3.
Supraventricular Tachycardia
11.6.13.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
11.6.13.2.5.
Wolff-Parkinson-White Syndrome (WPW)
11.6.13.2.6.
Bradycardia and Arrhythmias
11.6.13.2.7.
Others
11.6.13.3.
Canada Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
11.6.13.3.1.
Hospitals
11.6.13.3.2.
Clinics
11.6.13.3.3.
Ambulatory Care Centers
11.6.13.3.4.
Diagnostic Centers
11.6.13.3.5.
Others
11.6.14.
Mexico
11.6.14.1.
Mexico Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
11.6.14.1.1.
Access Device
11.6.14.1.1.1.
Steerable Sheath
11.6.14.1.1.2.
Transseptal Delivery System
11.6.14.1.2.
Accessories
11.6.14.1.2.1.
Pericardiocentesis Kits
11.6.14.1.2.2.
Others
11.6.14.1.3.
Cardiac Resynchronization Therapy
Defibrillator
11.6.14.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
11.6.14.1.5.
Capital Equipment: Diagnostic
11.6.14.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
11.6.14.1.5.2.
EP Recording System
11.6.14.1.6.
Capital Equipment: Mapping and
Navigation
11.6.14.1.7.
Capital Equipment: Therapy
11.6.14.1.8.
Catheters
11.6.14.1.8.1.
Ablation
11.6.14.1.8.2.
Diagnostic
11.6.14.1.8.3.
Mapping
11.6.14.1.9.
Defibrillators (ICD)
11.6.14.1.10.
Leads
11.6.14.1.11.
Left Atrial Appendage Closure
11.6.14.1.12.
Pace Makers
11.6.14.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
11.6.14.1.14.
Stimulators, WPI amplifiers and
isolators
11.6.14.1.15.
Others
11.6.14.2.
Mexico Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
11.6.14.2.1.
Aortic stenosis and mitral
regurgitation
11.6.14.2.2.
Atrial Fibrillation (AF)
11.6.14.2.3.
Supraventricular Tachycardia
11.6.14.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
11.6.14.2.5.
Wolff-Parkinson-White Syndrome (WPW)
11.6.14.2.6.
Bradycardia and Arrhythmias
11.6.14.2.7.
Others
11.6.14.3.
Mexico Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
11.6.14.3.1.
Hospitals
11.6.14.3.2.
Clinics
11.6.14.3.3.
Ambulatory Care Centers
11.6.14.3.4.
Diagnostic Centers
11.6.14.3.5.
Others
11.6.15.
Rest of North America
11.6.15.1.
Rest of North America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
11.6.15.1.1.
Access Device
11.6.15.1.1.1.
Steerable Sheath
11.6.15.1.1.2.
Transseptal Delivery System
11.6.15.1.2.
Accessories
11.6.15.1.2.1.
Pericardiocentesis Kits
11.6.15.1.2.2.
Others
11.6.15.1.3.
Cardiac Resynchronization Therapy
Defibrillator
11.6.15.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
11.6.15.1.5.
Capital Equipment: Diagnostic
11.6.15.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
11.6.15.1.5.2.
EP Recording System
11.6.15.1.6.
Capital Equipment: Mapping and
Navigation
11.6.15.1.7.
Capital Equipment: Therapy
11.6.15.1.8.
Catheters
11.6.15.1.8.1.
Ablation
11.6.15.1.8.2.
Diagnostic
11.6.15.1.8.3.
Mapping
11.6.15.1.9.
Defibrillators (ICD)
11.6.15.1.10.
Leads
11.6.15.1.11.
Left Atrial Appendage Closure
11.6.15.1.12.
Pace Makers
11.6.15.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
11.6.15.1.14.
Stimulators, WPI amplifiers and isolators
11.6.15.1.15.
Others
11.6.15.2.
Rest of North America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
11.6.15.2.1.
Aortic stenosis and mitral
regurgitation
11.6.15.2.2.
Atrial Fibrillation (AF)
11.6.15.2.3.
Supraventricular Tachycardia
11.6.15.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
11.6.15.2.5.
Wolff-Parkinson-White Syndrome (WPW)
11.6.15.2.6.
Bradycardia and Arrhythmias
11.6.15.2.7.
Others
11.6.15.3.
Rest of North America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By End-User
11.6.15.3.1.
Hospitals
11.6.15.3.2.
Clinics
11.6.15.3.3.
Ambulatory Care Centers
11.6.15.3.4.
Diagnostic Centers
11.6.15.3.5.
Others
11.7.
Key Segment for Channeling Investments
11.7.12.
By Country
11.7.13.
By Offerings
11.7.14.
By Clinical Indication
11.7.15.
By End-User
12 Europe Electrophysiology (EP) Devices Market Analysis and
Forecasts, 2020 – 2030
12.2.
Overview
12.2.12.
Europe Electrophysiology (EP) Devices
Market Revenue (US$ Mn)
12.3.
Europe Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.3.12.
Access Device
12.3.12.1.
Steerable Sheath
12.3.12.2.
Transseptal Delivery System
12.3.13.
Accessories
12.3.13.1.
Pericardiocentesis Kits
12.3.13.2.
Others
12.3.14.
Cardiac Resynchronization Therapy
Defibrillator
12.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.3.16.
Capital Equipment: Diagnostic
12.3.16.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.3.16.2.
EP Recording System
12.3.17.
Capital Equipment: Mapping and
Navigation
12.3.18.
Capital Equipment: Therapy
12.3.19.
Catheters
12.3.19.1.
Ablation
12.3.19.2.
Diagnostic
12.3.19.3.
Mapping
12.3.20.
Defibrillators (ICD)
12.3.21.
Leads
12.3.22.
Left Atrial Appendage Closure
12.3.23.
Pace Makers
12.3.24.
Remote Patient Monitoring and
Diagnostic Monitoring
12.3.25.
Stimulators, WPI amplifiers and
isolators
12.3.26.
Others
12.4.
Europe Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.4.12.
Aortic stenosis and mitral
regurgitation
12.4.13.
Atrial Fibrillation (AF)
12.4.14.
Supraventricular Tachycardia
12.4.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.4.16.
Wolff-Parkinson-White Syndrome (WPW)
12.4.17.
Bradycardia and Arrhythmias
12.4.18.
Others
12.5.
Europe Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.5.12.
Hospitals
12.5.13.
Clinics
12.5.14.
Ambulatory Care Centers
12.5.15.
Diagnostic Centers
12.5.16.
Others
12.6.
Europe Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Country
12.6.12.
France
12.6.12.1.
France Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.12.1.1.
Access Device
12.6.12.1.1.1.
Steerable Sheath
12.6.12.1.1.2.
Transseptal Delivery System
12.6.12.1.2.
Accessories
12.6.12.1.2.1.
Pericardiocentesis Kits
12.6.12.1.2.2.
Others
12.6.12.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.12.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.12.1.5.
Capital Equipment: Diagnostic
12.6.12.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.12.1.5.2.
EP Recording System
12.6.12.1.6.
Capital Equipment: Mapping and
Navigation
12.6.12.1.7.
Capital Equipment: Therapy
12.6.12.1.8.
Catheters
12.6.12.1.8.1.
Ablation
12.6.12.1.8.2.
Diagnostic
12.6.12.1.8.3.
Mapping
12.6.12.1.9.
Defibrillators (ICD)
12.6.12.1.10.
Leads
12.6.12.1.11.
Left Atrial Appendage Closure
12.6.12.1.12.
Pace Makers
12.6.12.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.12.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.12.1.15.
Others
12.6.12.2.
France Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.12.2.1.
Aortic stenosis and mitral
regurgitation
12.6.12.2.2.
Atrial Fibrillation (AF)
12.6.12.2.3.
Supraventricular Tachycardia
12.6.12.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.12.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.12.2.6.
Bradycardia and Arrhythmias
12.6.12.2.7.
Others
12.6.12.3.
France Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.12.3.1.
Hospitals
12.6.12.3.2.
Clinics
12.6.12.3.3.
Ambulatory Care Centers
12.6.12.3.4.
Diagnostic Centers
12.6.12.3.5.
Others
12.6.13.
The UK
12.6.13.1.
The UK Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.13.1.1.
Access Device
12.6.13.1.1.1.
Steerable Sheath
12.6.13.1.1.2.
Transseptal Delivery System
12.6.13.1.2.
Accessories
12.6.13.1.2.1.
Pericardiocentesis Kits
12.6.13.1.2.2.
Others
12.6.13.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.13.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.13.1.5.
Capital Equipment: Diagnostic
12.6.13.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.13.1.5.2.
EP Recording System
12.6.13.1.6.
Capital Equipment: Mapping and
Navigation
12.6.13.1.7.
Capital Equipment: Therapy
12.6.13.1.8.
Catheters
12.6.13.1.8.1.
Ablation
12.6.13.1.8.2.
Diagnostic
12.6.13.1.8.3.
Mapping
12.6.13.1.9.
Defibrillators (ICD)
12.6.13.1.10.
Leads
12.6.13.1.11.
Left Atrial Appendage Closure
12.6.13.1.12.
Pace Makers
12.6.13.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.13.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.13.1.15.
Others
12.6.13.2.
The UK Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.13.2.1.
Aortic stenosis and mitral
regurgitation
12.6.13.2.2.
Atrial Fibrillation (AF)
12.6.13.2.3.
Supraventricular Tachycardia
12.6.13.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.13.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.13.2.6.
Bradycardia and Arrhythmias
12.6.13.2.7.
Others
12.6.13.3.
The UK Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.13.3.1.
Hospitals
12.6.13.3.2.
Clinics
12.6.13.3.3.
Ambulatory Care Centers
12.6.13.3.4.
Diagnostic Centers
12.6.13.3.5.
Others
12.6.14.
Spain
12.6.14.1.
Spain Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.14.1.1.
Access Device
12.6.14.1.1.1.
Steerable Sheath
12.6.14.1.1.2.
Transseptal Delivery System
12.6.14.1.2.
Accessories
12.6.14.1.2.1.
Pericardiocentesis Kits
12.6.14.1.2.2.
Others
12.6.14.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.14.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.14.1.5.
Capital Equipment: Diagnostic
12.6.14.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.14.1.5.2.
EP Recording System
12.6.14.1.6.
Capital Equipment: Mapping and
Navigation
12.6.14.1.7.
Capital Equipment: Therapy
12.6.14.1.8.
Catheters
12.6.14.1.8.1.
Ablation
12.6.14.1.8.2.
Diagnostic
12.6.14.1.8.3.
Mapping
12.6.14.1.9.
Defibrillators (ICD)
12.6.14.1.10.
Leads
12.6.14.1.11.
Left Atrial Appendage Closure
12.6.14.1.12.
Pace Makers
12.6.14.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.14.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.14.1.15.
Others
12.6.14.2.
Spain Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.14.2.1.
Aortic stenosis and mitral
regurgitation
12.6.14.2.2.
Atrial Fibrillation (AF)
12.6.14.2.3.
Supraventricular Tachycardia
12.6.14.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.14.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.14.2.6.
Bradycardia and Arrhythmias
12.6.14.2.7.
Others
12.6.14.3.
Spain Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.14.3.1.
Hospitals
12.6.14.3.2.
Clinics
12.6.14.3.3.
Ambulatory Care Centers
12.6.14.3.4.
Diagnostic Centers
12.6.14.3.5.
Others
12.6.15.
Germany
12.6.15.1.
Germany Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.15.1.1.
Access Device
12.6.15.1.1.1.
Steerable Sheath
12.6.15.1.1.2.
Transseptal Delivery System
12.6.15.1.2.
Accessories
12.6.15.1.2.1.
Pericardiocentesis Kits
12.6.15.1.2.2.
Others
12.6.15.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.15.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.15.1.5.
Capital Equipment: Diagnostic
12.6.15.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.15.1.5.2.
EP Recording System
12.6.15.1.6.
Capital Equipment: Mapping and
Navigation
12.6.15.1.7.
Capital Equipment: Therapy
12.6.15.1.8.
Catheters
12.6.15.1.8.1.
Ablation
12.6.15.1.8.2.
Diagnostic
12.6.15.1.8.3.
Mapping
12.6.15.1.9.
Defibrillators (ICD)
12.6.15.1.10.
Leads
12.6.15.1.11.
Left Atrial Appendage Closure
12.6.15.1.12.
Pace Makers
12.6.15.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.15.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.15.1.15.
Others
12.6.15.2.
Germany Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.15.2.1.
Aortic stenosis and mitral
regurgitation
12.6.15.2.2.
Atrial Fibrillation (AF)
12.6.15.2.3.
Supraventricular Tachycardia
12.6.15.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.15.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.15.2.6.
Bradycardia and Arrhythmias
12.6.15.2.7.
Others
12.6.15.3.
Germany Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.15.3.1.
Hospitals
12.6.15.3.2.
Clinics
12.6.15.3.3.
Ambulatory Care Centers
12.6.15.3.4.
Diagnostic Centers
12.6.15.3.5.
Others
12.6.16.
Italy
12.6.16.1.
Italy Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.16.1.1.
Access Device
12.6.16.1.1.1.
Steerable Sheath
12.6.16.1.1.2.
Transseptal Delivery System
12.6.16.1.2.
Accessories
12.6.16.1.2.1.
Pericardiocentesis Kits
12.6.16.1.2.2.
Others
12.6.16.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.16.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.16.1.5.
Capital Equipment: Diagnostic
12.6.16.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.16.1.5.2.
EP Recording System
12.6.16.1.6.
Capital Equipment: Mapping and
Navigation
12.6.16.1.7.
Capital Equipment: Therapy
12.6.16.1.8.
Catheters
12.6.16.1.8.1.
Ablation
12.6.16.1.8.2.
Diagnostic
12.6.16.1.8.3.
Mapping
12.6.16.1.9.
Defibrillators (ICD)
12.6.16.1.10.
Leads
12.6.16.1.11.
Left Atrial Appendage Closure
12.6.16.1.12.
Pace Makers
12.6.16.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.16.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.16.1.15.
Others
12.6.16.2.
Italy Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.16.2.1.
Aortic stenosis and mitral
regurgitation
12.6.16.2.2.
Atrial Fibrillation (AF)
12.6.16.2.3.
Supraventricular Tachycardia
12.6.16.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.16.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.16.2.6.
Bradycardia and Arrhythmias
12.6.16.2.7.
Others
12.6.16.3.
Italy Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.16.3.1.
Hospitals
12.6.16.3.2.
Clinics
12.6.16.3.3.
Ambulatory Care Centers
12.6.16.3.4.
Diagnostic Centers
12.6.16.3.5.
Others
12.6.17.
Nordic Countries
12.6.17.1.
Nordic Countries Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.17.1.1.
Access Device
12.6.17.1.1.1.
Steerable Sheath
12.6.17.1.1.2.
Transseptal Delivery System
12.6.17.1.2.
Accessories
12.6.17.1.2.1.
Pericardiocentesis Kits
12.6.17.1.2.2.
Others
12.6.17.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.17.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.17.1.5.
Capital Equipment: Diagnostic
12.6.17.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.17.1.5.2.
EP Recording System
12.6.17.1.6.
Capital Equipment: Mapping and
Navigation
12.6.17.1.7.
Capital Equipment: Therapy
12.6.17.1.8.
Catheters
12.6.17.1.8.1.
Ablation
12.6.17.1.8.2.
Diagnostic
12.6.17.1.8.3.
Mapping
12.6.17.1.9.
Defibrillators (ICD)
12.6.17.1.10.
Leads
12.6.17.1.11.
Left Atrial Appendage Closure
12.6.17.1.12.
Pace Makers
12.6.17.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.17.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.17.1.15.
Others
12.6.17.2.
Nordic Countries Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.17.2.1.
Aortic stenosis and mitral
regurgitation
12.6.17.2.2.
Atrial Fibrillation (AF)
12.6.17.2.3.
Supraventricular Tachycardia
12.6.17.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.17.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.17.2.6.
Bradycardia and Arrhythmias
12.6.17.2.7.
Others
12.6.17.3.
Nordic Countries Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.17.3.1.
Hospitals
12.6.17.3.2.
Clinics
12.6.17.3.3.
Ambulatory Care Centers
12.6.17.3.4.
Diagnostic Centers
12.6.17.3.5.
Others
12.6.17.4.
Nordic Countries Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
12.6.17.4.1.
Denmark
12.6.17.4.2.
Finland
12.6.17.4.3.
Iceland
12.6.17.4.4.
Sweden
12.6.17.4.5.
Norway
12.6.18.
Benelux Union
12.6.18.1.
Benelux Union Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.18.1.1.
Access Device
12.6.18.1.1.1.
Steerable Sheath
12.6.18.1.1.2.
Transseptal Delivery System
12.6.18.1.2.
Accessories
12.6.18.1.2.1.
Pericardiocentesis Kits
12.6.18.1.2.2.
Others
12.6.18.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.18.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.18.1.5.
Capital Equipment: Diagnostic
12.6.18.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.18.1.5.2.
EP Recording System
12.6.18.1.6.
Capital Equipment: Mapping and
Navigation
12.6.18.1.7.
Capital Equipment: Therapy
12.6.18.1.8.
Catheters
12.6.18.1.8.1.
Ablation
12.6.18.1.8.2.
Diagnostic
12.6.18.1.8.3.
Mapping
12.6.18.1.9.
Defibrillators (ICD)
12.6.18.1.10.
Leads
12.6.18.1.11.
Left Atrial Appendage Closure
12.6.18.1.12.
Pace Makers
12.6.18.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.18.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.18.1.15.
Others
12.6.18.2.
Benelux Union Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.18.2.1.
Aortic stenosis and mitral
regurgitation
12.6.18.2.2.
Atrial Fibrillation (AF)
12.6.18.2.3.
Supraventricular Tachycardia
12.6.18.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.18.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.18.2.6.
Bradycardia and Arrhythmias
12.6.18.2.7.
Others
12.6.18.3.
Benelux Union Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.18.3.1.
Hospitals
12.6.18.3.2.
Clinics
12.6.18.3.3.
Ambulatory Care Centers
12.6.18.3.4.
Diagnostic Centers
12.6.18.3.5.
Others
12.6.18.4.
Benelux Union Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
12.6.18.4.1.
Belgium
12.6.18.4.2.
The Netherlands
12.6.18.4.3.
Luxembourg
12.6.19.
Rest of Europe
12.6.19.1.
Rest of Europe Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
12.6.19.1.1.
Access Device
12.6.19.1.1.1.
Steerable Sheath
12.6.19.1.1.2.
Transseptal Delivery System
12.6.19.1.2.
Accessories
12.6.19.1.2.1.
Pericardiocentesis Kits
12.6.19.1.2.2.
Others
12.6.19.1.3.
Cardiac Resynchronization Therapy
Defibrillator
12.6.19.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
12.6.19.1.5.
Capital Equipment: Diagnostic
12.6.19.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
12.6.19.1.5.2.
EP Recording System
12.6.19.1.6.
Capital Equipment: Mapping and
Navigation
12.6.19.1.7.
Capital Equipment: Therapy
12.6.19.1.8.
Catheters
12.6.19.1.8.1.
Ablation
12.6.19.1.8.2.
Diagnostic
12.6.19.1.8.3.
Mapping
12.6.19.1.9.
Defibrillators (ICD)
12.6.19.1.10.
Leads
12.6.19.1.11.
Left Atrial Appendage Closure
12.6.19.1.12.
Pace Makers
12.6.19.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
12.6.19.1.14.
Stimulators, WPI amplifiers and
isolators
12.6.19.1.15.
Others
12.6.19.2.
Rest of Europe Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
12.6.19.2.1.
Aortic stenosis and mitral
regurgitation
12.6.19.2.2.
Atrial Fibrillation (AF)
12.6.19.2.3.
Supraventricular Tachycardia
12.6.19.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
12.6.19.2.5.
Wolff-Parkinson-White Syndrome (WPW)
12.6.19.2.6.
Bradycardia and Arrhythmias
12.6.19.2.7.
Others
12.6.19.3.
Rest of Europe Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
12.6.19.3.1.
Hospitals
12.6.19.3.2.
Clinics
12.6.19.3.3.
Ambulatory Care Centers
12.6.19.3.4.
Diagnostic Centers
12.6.19.3.5.
Others
12.7.
Key Segment for Channeling Investments
12.7.12.
By Country
12.7.13.
By Offerings
12.7.14.
By Clinical Indication
12.7.15.
By End-User
13 Asia Pacific Electrophysiology (EP) Devices Market Analysis
and Forecasts, 2020 – 2030
13.2.
Overview
13.2.12.
Asia Pacific Electrophysiology (EP)
Devices Market Revenue (US$ Mn)
13.3.
Asia Pacific Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.3.12.
Access Device
13.3.12.1.
Steerable Sheath
13.3.12.2.
Transseptal Delivery System
13.3.13.
Accessories
13.3.13.1.
Pericardiocentesis Kits
13.3.13.2.
Others
13.3.14.
Cardiac Resynchronization Therapy
Defibrillator
13.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.3.16.
Capital Equipment: Diagnostic
13.3.16.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.3.16.2.
EP Recording System
13.3.17.
Capital Equipment: Mapping and
Navigation
13.3.18.
Capital Equipment: Therapy
13.3.19.
Catheters
13.3.19.1.
Ablation
13.3.19.2.
Diagnostic
13.3.19.3.
Mapping
13.3.20.
Defibrillators (ICD)
13.3.21.
Leads
13.3.22.
Left Atrial Appendage Closure
13.3.23.
Pace Makers
13.3.24.
Remote Patient Monitoring and
Diagnostic Monitoring
13.3.25.
Stimulators, WPI amplifiers and
isolators
13.3.26.
Others
13.4.
Asia Pacific Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.4.12.
Aortic stenosis and mitral
regurgitation
13.4.13.
Atrial Fibrillation (AF)
13.4.14.
Supraventricular Tachycardia
13.4.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.4.16.
Wolff-Parkinson-White Syndrome (WPW)
13.4.17.
Bradycardia and Arrhythmias
13.4.18.
Others
13.5.
Asia Pacific Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.5.12.
Hospitals
13.5.13.
Clinics
13.5.14.
Ambulatory Care Centers
13.5.15.
Diagnostic Centers
13.5.16.
Others
13.6.
Asia Pacific Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
13.6.12.
China
13.6.12.1.
China Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.12.1.1.
Access Device
13.6.12.1.1.1.
Steerable Sheath
13.6.12.1.1.2.
Transseptal Delivery System
13.6.12.1.2.
Accessories
13.6.12.1.2.1.
Pericardiocentesis Kits
13.6.12.1.2.2.
Others
13.6.12.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.12.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.12.1.5.
Capital Equipment: Diagnostic
13.6.12.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.12.1.5.2.
EP Recording System
13.6.12.1.6.
Capital Equipment: Mapping and
Navigation
13.6.12.1.7.
Capital Equipment: Therapy
13.6.12.1.8.
Catheters
13.6.12.1.8.1.
Ablation
13.6.12.1.8.2.
Diagnostic
13.6.12.1.8.3.
Mapping
13.6.12.1.9.
Defibrillators (ICD)
13.6.12.1.10.
Leads
13.6.12.1.11.
Left Atrial Appendage Closure
13.6.12.1.12.
Pace Makers
13.6.12.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.12.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.12.1.15.
Others
13.6.12.2.
China Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.12.2.1.
Aortic stenosis and mitral
regurgitation
13.6.12.2.2.
Atrial Fibrillation (AF)
13.6.12.2.3.
Supraventricular Tachycardia
13.6.12.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.12.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.12.2.6.
Bradycardia and Arrhythmias
13.6.12.2.7.
Others
13.6.12.3.
China Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.12.3.1.
Hospitals
13.6.12.3.2.
Clinics
13.6.12.3.3.
Ambulatory Care Centers
13.6.12.3.4.
Diagnostic Centers
13.6.12.3.5.
Others
13.6.13.
Japan
13.6.13.1.
Japan Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.13.1.1.
Access Device
13.6.13.1.1.1.
Steerable Sheath
13.6.13.1.1.2.
Transseptal Delivery System
13.6.13.1.2.
Accessories
13.6.13.1.2.1.
Pericardiocentesis Kits
13.6.13.1.2.2.
Others
13.6.13.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.13.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.13.1.5.
Capital Equipment: Diagnostic
13.6.13.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.13.1.5.2.
EP Recording System
13.6.13.1.6.
Capital Equipment: Mapping and
Navigation
13.6.13.1.7.
Capital Equipment: Therapy
13.6.13.1.8.
Catheters
13.6.13.1.8.1.
Ablation
13.6.13.1.8.2.
Diagnostic
13.6.13.1.8.3.
Mapping
13.6.13.1.9.
Defibrillators (ICD)
13.6.13.1.10.
Leads
13.6.13.1.11.
Left Atrial Appendage Closure
13.6.13.1.12.
Pace Makers
13.6.13.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.13.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.13.1.15.
Others
13.6.13.2.
Japan Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.13.2.1.
Aortic stenosis and mitral
regurgitation
13.6.13.2.2.
Atrial Fibrillation (AF)
13.6.13.2.3.
Supraventricular Tachycardia
13.6.13.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.13.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.13.2.6.
Bradycardia and Arrhythmias
13.6.13.2.7.
Others
13.6.13.3.
Japan Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.13.3.1.
Hospitals
13.6.13.3.2.
Clinics
13.6.13.3.3.
Ambulatory Care Centers
13.6.13.3.4.
Diagnostic Centers
13.6.13.3.5.
Others
13.6.14.
India
13.6.14.1.
India Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.14.1.1.
Access Device
13.6.14.1.1.1.
Steerable Sheath
13.6.14.1.1.2.
Transseptal Delivery System
13.6.14.1.2.
Accessories
13.6.14.1.2.1.
Pericardiocentesis Kits
13.6.14.1.2.2.
Others
13.6.14.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.14.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.14.1.5.
Capital Equipment: Diagnostic
13.6.14.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.14.1.5.2.
EP Recording System
13.6.14.1.6.
Capital Equipment: Mapping and
Navigation
13.6.14.1.7.
Capital Equipment: Therapy
13.6.14.1.8.
Catheters
13.6.14.1.8.1.
Ablation
13.6.14.1.8.2.
Diagnostic
13.6.14.1.8.3.
Mapping
13.6.14.1.9.
Defibrillators (ICD)
13.6.14.1.10.
Leads
13.6.14.1.11.
Left Atrial Appendage Closure
13.6.14.1.12.
Pace Makers
13.6.14.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.14.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.14.1.15.
Others
13.6.14.2.
India Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.14.2.1.
Aortic stenosis and mitral
regurgitation
13.6.14.2.2.
Atrial Fibrillation (AF)
13.6.14.2.3.
Supraventricular Tachycardia
13.6.14.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.14.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.14.2.6.
Bradycardia and Arrhythmias
13.6.14.2.7.
Others
13.6.14.3.
India Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.14.3.1.
Hospitals
13.6.14.3.2.
Clinics
13.6.14.3.3.
Ambulatory Care Centers
13.6.14.3.4.
Diagnostic Centers
13.6.14.3.5.
Others
13.6.15.
New Zealand
13.6.15.1.
New Zealand Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.15.1.1.
Access Device
13.6.15.1.1.1.
Steerable Sheath
13.6.15.1.1.2.
Transseptal Delivery System
13.6.15.1.2.
Accessories
13.6.15.1.2.1.
Pericardiocentesis Kits
13.6.15.1.2.2.
Others
13.6.15.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.15.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.15.1.5.
Capital Equipment: Diagnostic
13.6.15.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.15.1.5.2.
EP Recording System
13.6.15.1.6.
Capital Equipment: Mapping and
Navigation
13.6.15.1.7.
Capital Equipment: Therapy
13.6.15.1.8.
Catheters
13.6.15.1.8.1.
Ablation
13.6.15.1.8.2.
Diagnostic
13.6.15.1.8.3.
Mapping
13.6.15.1.9.
Defibrillators (ICD)
13.6.15.1.10.
Leads
13.6.15.1.11.
Left Atrial Appendage Closure
13.6.15.1.12.
Pace Makers
13.6.15.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.15.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.15.1.15.
Others
13.6.15.2.
New Zealand Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.15.2.1.
Aortic stenosis and mitral
regurgitation
13.6.15.2.2.
Atrial Fibrillation (AF)
13.6.15.2.3.
Supraventricular Tachycardia
13.6.15.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.15.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.15.2.6.
Bradycardia and Arrhythmias
13.6.15.2.7.
Others
13.6.15.3.
New Zealand Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.15.3.1.
Hospitals
13.6.15.3.2.
Clinics
13.6.15.3.3.
Ambulatory Care Centers
13.6.15.3.4.
Diagnostic Centers
13.6.15.3.5.
Others
13.6.16.
Australia
13.6.16.1.
Australia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.16.1.1.
Access Device
13.6.16.1.1.1.
Steerable Sheath
13.6.16.1.1.2.
Transseptal Delivery System
13.6.16.1.2.
Accessories
13.6.16.1.2.1.
Pericardiocentesis Kits
13.6.16.1.2.2.
Others
13.6.16.1.3.
Cardiac Resynchronization Therapy Defibrillator
13.6.16.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.16.1.5.
Capital Equipment: Diagnostic
13.6.16.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.16.1.5.2.
EP Recording System
13.6.16.1.6.
Capital Equipment: Mapping and
Navigation
13.6.16.1.7.
Capital Equipment: Therapy
13.6.16.1.8.
Catheters
13.6.16.1.8.1.
Ablation
13.6.16.1.8.2.
Diagnostic
13.6.16.1.8.3.
Mapping
13.6.16.1.9.
Defibrillators (ICD)
13.6.16.1.10.
Leads
13.6.16.1.11.
Left Atrial Appendage Closure
13.6.16.1.12.
Pace Makers
13.6.16.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.16.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.16.1.15.
Others
13.6.16.2.
Australia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.16.2.1.
Aortic stenosis and mitral
regurgitation
13.6.16.2.2.
Atrial Fibrillation (AF)
13.6.16.2.3.
Supraventricular Tachycardia
13.6.16.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.16.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.16.2.6.
Bradycardia and Arrhythmias
13.6.16.2.7.
Others
13.6.16.3.
Australia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.16.3.1.
Hospitals
13.6.16.3.2.
Clinics
13.6.16.3.3.
Ambulatory Care Centers
13.6.16.3.4.
Diagnostic Centers
13.6.16.3.5.
Others
13.6.17.
South Korea
13.6.17.1.
South Korea Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.17.1.1.
Access Device
13.6.17.1.1.1.
Steerable Sheath
13.6.17.1.1.2.
Transseptal Delivery System
13.6.17.1.2.
Accessories
13.6.17.1.2.1.
Pericardiocentesis Kits
13.6.17.1.2.2.
Others
13.6.17.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.17.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.17.1.5.
Capital Equipment: Diagnostic
13.6.17.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.17.1.5.2.
EP Recording System
13.6.17.1.6.
Capital Equipment: Mapping and
Navigation
13.6.17.1.7.
Capital Equipment: Therapy
13.6.17.1.8.
Catheters
13.6.17.1.8.1.
Ablation
13.6.17.1.8.2.
Diagnostic
13.6.17.1.8.3.
Mapping
13.6.17.1.9.
Defibrillators (ICD)
13.6.17.1.10.
Leads
13.6.17.1.11.
Left Atrial Appendage Closure
13.6.17.1.12.
Pace Makers
13.6.17.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.17.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.17.1.15.
Others
13.6.17.2.
South Korea Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.17.2.1.
Aortic stenosis and mitral
regurgitation
13.6.17.2.2.
Atrial Fibrillation (AF)
13.6.17.2.3.
Supraventricular Tachycardia
13.6.17.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.17.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.17.2.6.
Bradycardia and Arrhythmias
13.6.17.2.7.
Others
13.6.17.3.
South Korea Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.17.3.1.
Hospitals
13.6.17.3.2.
Clinics
13.6.17.3.3.
Ambulatory Care Centers
13.6.17.3.4.
Diagnostic Centers
13.6.17.3.5.
Others
13.6.18.
Southeast Asia
13.6.18.1.
Southeast Asia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.18.1.1.
Access Device
13.6.18.1.1.1.
Steerable Sheath
13.6.18.1.1.2.
Transseptal Delivery System
13.6.18.1.2.
Accessories
13.6.18.1.2.1.
Pericardiocentesis Kits
13.6.18.1.2.2.
Others
13.6.18.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.18.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.18.1.5.
Capital Equipment: Diagnostic
13.6.18.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.18.1.5.2.
EP Recording System
13.6.18.1.6.
Capital Equipment: Mapping and
Navigation
13.6.18.1.7.
Capital Equipment: Therapy
13.6.18.1.8.
Catheters
13.6.18.1.8.1.
Ablation
13.6.18.1.8.2.
Diagnostic
13.6.18.1.8.3.
Mapping
13.6.18.1.9.
Defibrillators (ICD)
13.6.18.1.10.
Leads
13.6.18.1.11.
Left Atrial Appendage Closure
13.6.18.1.12.
Pace Makers
13.6.18.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.18.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.18.1.15.
Others
13.6.18.2.
Southeast Asia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.18.2.1.
Aortic stenosis and mitral
regurgitation
13.6.18.2.2.
Atrial Fibrillation (AF)
13.6.18.2.3.
Supraventricular Tachycardia
13.6.18.2.4.
Atrioventricular Nodal Re-entry Tachycardia
(AVNRT)
13.6.18.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.18.2.6.
Bradycardia and Arrhythmias
13.6.18.2.7.
Others
13.6.18.3.
Southeast Asia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.18.3.1.
Hospitals
13.6.18.3.2.
Clinics
13.6.18.3.3.
Ambulatory Care Centers
13.6.18.3.4.
Diagnostic Centers
13.6.18.3.5.
Others
13.6.18.4.
Southeast Asia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
13.6.18.4.1.
Indonesia
13.6.18.4.2.
Thailand
13.6.18.4.3.
Malaysia
13.6.18.4.4.
Singapore
13.6.18.4.5.
Rest of Southeast Asia
13.6.19.
Rest of Asia Pacific
13.6.19.1.
Rest of Asia Pacific Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
13.6.19.1.1.
Access Device
13.6.19.1.1.1.
Steerable Sheath
13.6.19.1.1.2.
Transseptal Delivery System
13.6.19.1.2.
Accessories
13.6.19.1.2.1.
Pericardiocentesis Kits
13.6.19.1.2.2.
Others
13.6.19.1.3.
Cardiac Resynchronization Therapy
Defibrillator
13.6.19.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
13.6.19.1.5.
Capital Equipment: Diagnostic
13.6.19.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
13.6.19.1.5.2.
EP Recording System
13.6.19.1.6.
Capital Equipment: Mapping and
Navigation
13.6.19.1.7.
Capital Equipment: Therapy
13.6.19.1.8.
Catheters
13.6.19.1.8.1.
Ablation
13.6.19.1.8.2.
Diagnostic
13.6.19.1.8.3.
Mapping
13.6.19.1.9.
Defibrillators (ICD)
13.6.19.1.10.
Leads
13.6.19.1.11.
Left Atrial Appendage Closure
13.6.19.1.12.
Pace Makers
13.6.19.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
13.6.19.1.14.
Stimulators, WPI amplifiers and
isolators
13.6.19.1.15.
Others
13.6.19.2.
Rest of Asia Pacific Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
13.6.19.2.1.
Aortic stenosis and mitral regurgitation
13.6.19.2.2.
Atrial Fibrillation (AF)
13.6.19.2.3.
Supraventricular Tachycardia
13.6.19.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
13.6.19.2.5.
Wolff-Parkinson-White Syndrome (WPW)
13.6.19.2.6.
Bradycardia and Arrhythmias
13.6.19.2.7.
Others
13.6.19.3.
Rest of Asia Pacific Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By End-User
13.6.19.3.1.
Hospitals
13.6.19.3.2.
Clinics
13.6.19.3.3.
Ambulatory Care Centers
13.6.19.3.4.
Diagnostic Centers
13.6.19.3.5.
Others
13.7.
Key Segment for Channeling Investments
13.7.12.
By Country
13.7.13.
By Offerings
13.7.14.
By Clinical Indication
13.7.15.
By End-User
14 Middle East and Africa Electrophysiology (EP) Devices Market
Analysis and Forecasts, 2020 – 2030
14.2.
Overview
14.2.12.
Middle East and Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn)
14.3.
Middle East and Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
Offerings
14.3.12.
Access Device
14.3.12.1.
Steerable Sheath
14.3.12.2.
Transseptal Delivery System
14.3.13.
Accessories
14.3.13.1.
Pericardiocentesis Kits
14.3.13.2.
Others
14.3.14.
Cardiac Resynchronization Therapy
Defibrillator
14.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.3.16.
Capital Equipment: Diagnostic
14.3.16.1.
Ultrasound Imaging System and Ultrasound
Imaging Catheter
14.3.16.2.
EP Recording System
14.3.17.
Capital Equipment: Mapping and
Navigation
14.3.18.
Capital Equipment: Therapy
14.3.19.
Catheters
14.3.19.1.
Ablation
14.3.19.2.
Diagnostic
14.3.19.3.
Mapping
14.3.20.
Defibrillators (ICD)
14.3.21.
Leads
14.3.22.
Left Atrial Appendage Closure
14.3.23.
Pace Makers
14.3.24.
Remote Patient Monitoring and Diagnostic
Monitoring
14.3.25.
Stimulators, WPI amplifiers and
isolators
14.3.26.
Others
14.4.
Middle East and Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
Clinical Indication
14.4.12.
Aortic stenosis and mitral
regurgitation
14.4.13.
Atrial Fibrillation (AF)
14.4.14.
Supraventricular Tachycardia
14.4.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.4.16.
Wolff-Parkinson-White Syndrome (WPW)
14.4.17.
Bradycardia and Arrhythmias
14.4.18.
Others
14.5.
Middle East and Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
End-User
14.5.12.
Hospitals
14.5.13.
Clinics
14.5.14.
Ambulatory Care Centers
14.5.15.
Diagnostic Centers
14.5.16.
Others
14.6.
Middle East and Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
Country
14.6.12.
Saudi Arabia
14.6.12.1.
Saudi Arabia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
14.6.12.1.1.
Access Device
14.6.12.1.1.1.
Steerable Sheath
14.6.12.1.1.2.
Transseptal Delivery System
14.6.12.1.2.
Accessories
14.6.12.1.2.1.
Pericardiocentesis Kits
14.6.12.1.2.2.
Others
14.6.12.1.3.
Cardiac Resynchronization Therapy
Defibrillator
14.6.12.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.12.1.5.
Capital Equipment: Diagnostic
14.6.12.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.12.1.5.2.
EP Recording System
14.6.12.1.6.
Capital Equipment: Mapping and
Navigation
14.6.12.1.7.
Capital Equipment: Therapy
14.6.12.1.8.
Catheters
14.6.12.1.8.1.
Ablation
14.6.12.1.8.2.
Diagnostic
14.6.12.1.8.3.
Mapping
14.6.12.1.9.
Defibrillators (ICD)
14.6.12.1.10.
Leads
14.6.12.1.11.
Left Atrial Appendage Closure
14.6.12.1.12.
Pace Makers
14.6.12.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.12.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.12.1.15.
Others
14.6.12.2.
Saudi Arabia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
14.6.12.2.1.
Aortic stenosis and mitral regurgitation
14.6.12.2.2.
Atrial Fibrillation (AF)
14.6.12.2.3.
Supraventricular Tachycardia
14.6.12.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.12.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.12.2.6.
Bradycardia and Arrhythmias
14.6.12.2.7.
Others
14.6.12.3.
Saudi Arabia Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
14.6.12.3.1.
Hospitals
14.6.12.3.2.
Clinics
14.6.12.3.3.
Ambulatory Care Centers
14.6.12.3.4.
Diagnostic Centers
14.6.12.3.5.
Others
14.6.13.
UAE
14.6.13.1.
UAE Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
14.6.13.1.1.
Access Device
14.6.13.1.1.1.
Steerable Sheath
14.6.13.1.1.2.
Transseptal Delivery System
14.6.13.1.2.
Accessories
14.6.13.1.2.1.
Pericardiocentesis Kits
14.6.13.1.2.2.
Others
14.6.13.1.3.
Cardiac Resynchronization Therapy
Defibrillator
14.6.13.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.13.1.5.
Capital Equipment: Diagnostic
14.6.13.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.13.1.5.2.
EP Recording System
14.6.13.1.6.
Capital Equipment: Mapping and
Navigation
14.6.13.1.7.
Capital Equipment: Therapy
14.6.13.1.8.
Catheters
14.6.13.1.8.1.
Ablation
14.6.13.1.8.2.
Diagnostic
14.6.13.1.8.3.
Mapping
14.6.13.1.9.
Defibrillators (ICD)
14.6.13.1.10.
Leads
14.6.13.1.11.
Left Atrial Appendage Closure
14.6.13.1.12.
Pace Makers
14.6.13.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.13.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.13.1.15.
Others
14.6.13.2.
UAE Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
14.6.13.2.1.
Aortic stenosis and mitral
regurgitation
14.6.13.2.2.
Atrial Fibrillation (AF)
14.6.13.2.3.
Supraventricular Tachycardia
14.6.13.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.13.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.13.2.6.
Bradycardia and Arrhythmias
14.6.13.2.7.
Others
14.6.13.3.
UAE Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
14.6.13.3.1.
Hospitals
14.6.13.3.2.
Clinics
14.6.13.3.3.
Ambulatory Care Centers
14.6.13.3.4.
Diagnostic Centers
14.6.13.3.5.
Others
14.6.14.
Egypt
14.6.14.1.
Egypt Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
14.6.14.1.1.
Access Device
14.6.14.1.1.1.
Steerable Sheath
14.6.14.1.1.2.
Transseptal Delivery System
14.6.14.1.2.
Accessories
14.6.14.1.2.1.
Pericardiocentesis Kits
14.6.14.1.2.2.
Others
14.6.14.1.3.
Cardiac Resynchronization Therapy
Defibrillator
14.6.14.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.14.1.5.
Capital Equipment: Diagnostic
14.6.14.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.14.1.5.2.
EP Recording System
14.6.14.1.6.
Capital Equipment: Mapping and
Navigation
14.6.14.1.7.
Capital Equipment: Therapy
14.6.14.1.8.
Catheters
14.6.14.1.8.1.
Ablation
14.6.14.1.8.2.
Diagnostic
14.6.14.1.8.3.
Mapping
14.6.14.1.9.
Defibrillators (ICD)
14.6.14.1.10.
Leads
14.6.14.1.11.
Left Atrial Appendage Closure
14.6.14.1.12.
Pace Makers
14.6.14.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.14.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.14.1.15.
Others
14.6.14.2.
Egypt Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
14.6.14.2.1.
Aortic stenosis and mitral
regurgitation
14.6.14.2.2.
Atrial Fibrillation (AF)
14.6.14.2.3.
Supraventricular Tachycardia
14.6.14.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.14.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.14.2.6.
Bradycardia and Arrhythmias
14.6.14.2.7.
Others
14.6.14.3.
Egypt Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
14.6.14.3.1.
Hospitals
14.6.14.3.2.
Clinics
14.6.14.3.3.
Ambulatory Care Centers
14.6.14.3.4.
Diagnostic Centers
14.6.14.3.5.
Others
14.6.15.
Kuwait
14.6.15.1.
Kuwait Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
14.6.15.1.1.
Access Device
14.6.15.1.1.1.
Steerable Sheath
14.6.15.1.1.2.
Transseptal Delivery System
14.6.15.1.2.
Accessories
14.6.15.1.2.1.
Pericardiocentesis Kits
14.6.15.1.2.2.
Others
14.6.15.1.3.
Cardiac Resynchronization Therapy
Defibrillator
14.6.15.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.15.1.5.
Capital Equipment: Diagnostic
14.6.15.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.15.1.5.2.
EP Recording System
14.6.15.1.6.
Capital Equipment: Mapping and
Navigation
14.6.15.1.7.
Capital Equipment: Therapy
14.6.15.1.8.
Catheters
14.6.15.1.8.1.
Ablation
14.6.15.1.8.2.
Diagnostic
14.6.15.1.8.3.
Mapping
14.6.15.1.9.
Defibrillators (ICD)
14.6.15.1.10.
Leads
14.6.15.1.11.
Left Atrial Appendage Closure
14.6.15.1.12.
Pace Makers
14.6.15.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.15.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.15.1.15.
Others
14.6.15.2.
Kuwait Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
14.6.15.2.1.
Aortic stenosis and mitral
regurgitation
14.6.15.2.2.
Atrial Fibrillation (AF)
14.6.15.2.3.
Supraventricular Tachycardia
14.6.15.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.15.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.15.2.6.
Bradycardia and Arrhythmias
14.6.15.2.7.
Others
14.6.15.3.
Kuwait Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
14.6.15.3.1.
Hospitals
14.6.15.3.2.
Clinics
14.6.15.3.3.
Ambulatory Care Centers
14.6.15.3.4.
Diagnostic Centers
14.6.15.3.5.
Others
14.6.16.
South Africa
14.6.16.1.
South Africa Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
14.6.16.1.1.
Access Device
14.6.16.1.1.1.
Steerable Sheath
14.6.16.1.1.2.
Transseptal Delivery System
14.6.16.1.2.
Accessories
14.6.16.1.2.1.
Pericardiocentesis Kits
14.6.16.1.2.2.
Others
14.6.16.1.3.
Cardiac Resynchronization Therapy
Defibrillator
14.6.16.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.16.1.5.
Capital Equipment: Diagnostic
14.6.16.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.16.1.5.2.
EP Recording System
14.6.16.1.6.
Capital Equipment: Mapping and
Navigation
14.6.16.1.7.
Capital Equipment: Therapy
14.6.16.1.8.
Catheters
14.6.16.1.8.1.
Ablation
14.6.16.1.8.2.
Diagnostic
14.6.16.1.8.3.
Mapping
14.6.16.1.9.
Defibrillators (ICD)
14.6.16.1.10.
Leads
14.6.16.1.11.
Left Atrial Appendage Closure
14.6.16.1.12.
Pace Makers
14.6.16.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.16.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.16.1.15.
Others
14.6.16.2.
South Africa Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
14.6.16.2.1.
Aortic stenosis and mitral
regurgitation
14.6.16.2.2.
Atrial Fibrillation (AF)
14.6.16.2.3.
Supraventricular Tachycardia
14.6.16.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.16.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.16.2.6.
Bradycardia and Arrhythmias
14.6.16.2.7.
Others
14.6.16.3.
South Africa Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
14.6.16.3.1.
Hospitals
14.6.16.3.2.
Clinics
14.6.16.3.3.
Ambulatory Care Centers
14.6.16.3.4.
Diagnostic Centers
14.6.16.3.5.
Others
14.6.17.
Rest of Middle East & Africa
14.6.17.1.
Rest of Middle East & Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
Offerings
14.6.17.1.1.
Access Device
14.6.17.1.1.1.
Steerable Sheath
14.6.17.1.1.2.
Transseptal Delivery System
14.6.17.1.2.
Accessories
14.6.17.1.2.1.
Pericardiocentesis Kits
14.6.17.1.2.2.
Others
14.6.17.1.3.
Cardiac Resynchronization Therapy Defibrillator
14.6.17.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
14.6.17.1.5.
Capital Equipment: Diagnostic
14.6.17.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
14.6.17.1.5.2.
EP Recording System
14.6.17.1.6.
Capital Equipment: Mapping and
Navigation
14.6.17.1.7.
Capital Equipment: Therapy
14.6.17.1.8.
Catheters
14.6.17.1.8.1.
Ablation
14.6.17.1.8.2.
Diagnostic
14.6.17.1.8.3.
Mapping
14.6.17.1.9.
Defibrillators (ICD)
14.6.17.1.10.
Leads
14.6.17.1.11.
Left Atrial Appendage Closure
14.6.17.1.12.
Pace Makers
14.6.17.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
14.6.17.1.14.
Stimulators, WPI amplifiers and
isolators
14.6.17.1.15.
Others
14.6.17.2.
Rest of Middle East & Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
Clinical Indication
14.6.17.2.1.
Aortic stenosis and mitral
regurgitation
14.6.17.2.2.
Atrial Fibrillation (AF)
14.6.17.2.3.
Supraventricular Tachycardia
14.6.17.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
14.6.17.2.5.
Wolff-Parkinson-White Syndrome (WPW)
14.6.17.2.6.
Bradycardia and Arrhythmias
14.6.17.2.7.
Others
14.6.17.3.
Rest of Middle East & Africa
Electrophysiology (EP) Devices Market Revenue (US$ Mn) and Forecasts, By
End-User
14.6.17.3.1.
Hospitals
14.6.17.3.2.
Clinics
14.6.17.3.3.
Ambulatory Care Centers
14.6.17.3.4.
Diagnostic Centers
14.6.17.3.5.
Others
14.7.
Key Segment for Channeling Investments
14.7.12.
By Country
14.7.13.
By Offerings
14.7.14.
By Clinical Indication
14.7.15.
By End-User
15 Latin America Electrophysiology (EP) Devices Market Analysis
and Forecasts, 2020 - 2030
15.2.
Overview
15.2.12.
Latin America Electrophysiology (EP)
Devices Market Revenue (US$ Mn)
15.3.
Latin America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
15.3.12.
Access Device
15.3.12.1.
Steerable Sheath
15.3.12.2.
Transseptal Delivery System
15.3.13.
Accessories
15.3.13.1.
Pericardiocentesis Kits
15.3.13.2.
Others
15.3.14.
Cardiac Resynchronization Therapy
Defibrillator
15.3.15.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
15.3.16.
Capital Equipment: Diagnostic
15.3.16.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
15.3.16.2.
EP Recording System
15.3.17.
Capital Equipment: Mapping and
Navigation
15.3.18.
Capital Equipment: Therapy
15.3.19.
Catheters
15.3.19.1.
Ablation
15.3.19.2.
Diagnostic
15.3.19.3.
Mapping
15.3.20.
Defibrillators (ICD)
15.3.21.
Leads
15.3.22.
Left Atrial Appendage Closure
15.3.23.
Pace Makers
15.3.24.
Remote Patient Monitoring and
Diagnostic Monitoring
15.3.25.
Stimulators, WPI amplifiers and
isolators
15.3.26.
Others
15.4.
Latin America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
15.4.12.
Aortic stenosis and mitral
regurgitation
15.4.13.
Atrial Fibrillation (AF)
15.4.14.
Supraventricular Tachycardia
15.4.15.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
15.4.16.
Wolff-Parkinson-White Syndrome (WPW)
15.4.17.
Bradycardia and Arrhythmias
15.4.18.
Others
15.5.
Latin America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
15.5.12.
Hospitals
15.5.13.
Clinics
15.5.14.
Ambulatory Care Centers
15.5.15.
Diagnostic Centers
15.5.16.
Others
15.6.
Latin America Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Country
15.6.12.
Brazil
15.6.12.1.
Brazil Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Offerings
15.6.12.1.1.
Access Device
15.6.12.1.1.1.
Steerable Sheath
15.6.12.1.1.2.
Transseptal Delivery System
15.6.12.1.2.
Accessories
15.6.12.1.2.1.
Pericardiocentesis Kits
15.6.12.1.2.2.
Others
15.6.12.1.3.
Cardiac Resynchronization Therapy
Defibrillator
15.6.12.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
15.6.12.1.5.
Capital Equipment: Diagnostic
15.6.12.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
15.6.12.1.5.2.
EP Recording System
15.6.12.1.6.
Capital Equipment: Mapping and
Navigation
15.6.12.1.7.
Capital Equipment: Therapy
15.6.12.1.8.
Catheters
15.6.12.1.8.1.
Ablation
15.6.12.1.8.2.
Diagnostic
15.6.12.1.8.3.
Mapping
15.6.12.1.9.
Defibrillators (ICD)
15.6.12.1.10.
Leads
15.6.12.1.11.
Left Atrial Appendage Closure
15.6.12.1.12.
Pace Makers
15.6.12.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
15.6.12.1.14.
Stimulators, WPI amplifiers and
isolators
15.6.12.1.15.
Others
15.6.12.2.
Brazil Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
15.6.12.2.1.
Aortic stenosis and mitral
regurgitation
15.6.12.2.2.
Atrial Fibrillation (AF)
15.6.12.2.3.
Supraventricular Tachycardia
15.6.12.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
15.6.12.2.5.
Wolff-Parkinson-White Syndrome (WPW)
15.6.12.2.6.
Bradycardia and Arrhythmias
15.6.12.2.7.
Others
15.6.12.3.
Brazil Electrophysiology (EP) Devices
Market Revenue (US$ Mn) and Forecasts, By End-User
15.6.12.3.1.
Hospitals
15.6.12.3.2.
Clinics
15.6.12.3.3.
Ambulatory Care Centers
15.6.12.3.4.
Diagnostic Centers
15.6.12.3.5.
Others
15.6.13.
Argentina
15.6.13.1.
Argentina Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
15.6.13.1.1.
Access Device
15.6.13.1.1.1.
Steerable Sheath
15.6.13.1.1.2.
Transseptal Delivery System
15.6.13.1.2.
Accessories
15.6.13.1.2.1.
Pericardiocentesis Kits
15.6.13.1.2.2.
Others
15.6.13.1.3.
Cardiac Resynchronization Therapy
Defibrillator
15.6.13.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
15.6.13.1.5.
Capital Equipment: Diagnostic
15.6.13.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
15.6.13.1.5.2.
EP Recording System
15.6.13.1.6.
Capital Equipment: Mapping and
Navigation
15.6.13.1.7.
Capital Equipment: Therapy
15.6.13.1.8.
Catheters
15.6.13.1.8.1.
Ablation
15.6.13.1.8.2.
Diagnostic
15.6.13.1.8.3.
Mapping
15.6.13.1.9.
Defibrillators (ICD)
15.6.13.1.10.
Leads
15.6.13.1.11.
Left Atrial Appendage Closure
15.6.13.1.12.
Pace Makers
15.6.13.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
15.6.13.1.14.
Stimulators, WPI amplifiers and
isolators
15.6.13.1.15.
Others
15.6.13.2.
Argentina Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
15.6.13.2.1.
Aortic stenosis and mitral
regurgitation
15.6.13.2.2.
Atrial Fibrillation (AF)
15.6.13.2.3.
Supraventricular Tachycardia
15.6.13.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
15.6.13.2.5.
Wolff-Parkinson-White Syndrome (WPW)
15.6.13.2.6.
Bradycardia and Arrhythmias
15.6.13.2.7.
Others
15.6.13.3.
Argentina Electrophysiology (EP)
Devices Market Revenue (US$ Mn) and Forecasts, By End-User
15.6.13.3.1.
Hospitals
15.6.13.3.2.
Clinics
15.6.13.3.3.
Ambulatory Care Centers
15.6.13.3.4.
Diagnostic Centers
15.6.13.3.5.
Others
15.6.14.
Rest of Latin America
15.6.14.1.
Rest of Latin America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Offerings
15.6.14.1.1.
Access Device
15.6.14.1.1.1.
Steerable Sheath
15.6.14.1.1.2.
Transseptal Delivery System
15.6.14.1.2.
Accessories
15.6.14.1.2.1.
Pericardiocentesis Kits
15.6.14.1.2.2.
Others
15.6.14.1.3.
Cardiac Resynchronization Therapy
Defibrillator
15.6.14.1.4.
Cardiac Resynchronization Therapy
Pacemakers (CRT-P)
15.6.14.1.5.
Capital Equipment: Diagnostic
15.6.14.1.5.1.
Ultrasound Imaging System and
Ultrasound Imaging Catheter
15.6.14.1.5.2.
EP Recording System
15.6.14.1.6.
Capital Equipment: Mapping and
Navigation
15.6.14.1.7.
Capital Equipment: Therapy
15.6.14.1.8.
Catheters
15.6.14.1.8.1.
Ablation
15.6.14.1.8.2.
Diagnostic
15.6.14.1.8.3.
Mapping
15.6.14.1.9.
Defibrillators (ICD)
15.6.14.1.10.
Leads
15.6.14.1.11.
Left Atrial Appendage Closure
15.6.14.1.12.
Pace Makers
15.6.14.1.13.
Remote Patient Monitoring and
Diagnostic Monitoring
15.6.14.1.14.
Stimulators, WPI amplifiers and
isolators
15.6.14.1.15.
Others
15.6.14.2.
Rest of Latin America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By Clinical Indication
15.6.14.2.1.
Aortic stenosis and mitral
regurgitation
15.6.14.2.2.
Atrial Fibrillation (AF)
15.6.14.2.3.
Supraventricular Tachycardia
15.6.14.2.4.
Atrioventricular Nodal Re-entry
Tachycardia (AVNRT)
15.6.14.2.5.
Wolff-Parkinson-White Syndrome (WPW)
15.6.14.2.6.
Bradycardia and Arrhythmias
15.6.14.2.7.
Others
15.6.14.3.
Rest of Latin America Electrophysiology
(EP) Devices Market Revenue (US$ Mn) and Forecasts, By End-User
15.6.14.3.1.
Hospitals
15.6.14.3.2.
Clinics
15.6.14.3.3.
Ambulatory Care Centers
15.6.14.3.4.
Diagnostic Centers
15.6.14.3.5.
Others
15.7.
Key Segment for Channeling Investments
15.7.12.
By Country
15.7.13.
By Offerings
15.7.14.
By Clinical Indication
15.7.15.
By End-User
16 Competitive Benchmarking
16.2.
Market Share Analysis, 2019
16.3.
Global Presence and Growth Strategies
16.3.12.
Mergers and Acquisitions
16.3.13.
Product Launches
16.3.14.
Investments Trends
16.3.15.
R&D Initiatives
17 Player Profiles
17.2.
Abbott
17.2.12.
Company Details
17.2.13.
Company Overview
17.2.14.
Product Offerings
17.2.15.
Key Developments
17.2.16.
Financial Analysis
17.2.17.
SWOT Analysis
17.2.18.
Business Strategies
17.3.
ADInstruments NZ Limited
17.3.12.
Company Details
17.3.13.
Company Overview
17.3.14.
Product Offerings
17.3.15.
Key Developments
17.3.16.
Financial Analysis
17.3.17.
SWOT Analysis
17.3.18.
Business Strategies
17.4.
BIOTRONIK SE & Co. KG
17.4.12.
Company Details
17.4.13.
Company Overview
17.4.14.
Product Offerings
17.4.15.
Key Developments
17.4.16.
Financial Analysis
17.4.17.
SWOT Analysis
17.4.18.
Business Strategies
17.5.
Boston Scientific Corporation
17.5.12.
Company Details
17.5.13.
Company Overview
17.5.14.
Product Offerings
17.5.15.
Key Developments
17.5.16.
Financial Analysis
17.5.17.
SWOT Analysis
17.5.18.
Business Strategies
17.6.
GENERAL ELECTRIC COMPANY
17.6.12.
Company Details
17.6.13.
Company Overview
17.6.14.
Product Offerings
17.6.15.
Key Developments
17.6.16.
Financial Analysis
17.6.17.
SWOT Analysis
17.6.18.
Business Strategies
17.7.
Koninklijke Philips N.V.
17.7.12.
Company Details
17.7.13.
Company Overview
17.7.14.
Product Offerings
17.7.15.
Key Developments
17.7.16.
Financial Analysis
17.7.17.
SWOT Analysis
17.7.18.
Business Strategies
17.8.
Medical Devices Business Services, Inc
17.8.12.
Company Details
17.8.13.
Company Overview
17.8.14.
Product Offerings
17.8.15.
Key Developments
17.8.16.
Financial Analysis
17.8.17.
SWOT Analysis
17.8.18.
Business Strategies
17.9.
Medtronic
17.9.12.
Company Details
17.9.13.
Company Overview
17.9.14.
Product Offerings
17.9.15.
Key Developments
17.9.16.
Financial Analysis
17.9.17.
SWOT Analysis
17.9.18.
Business Strategies
17.10.
MicroPort Scientific Corporation
17.10.12.
Company Details
17.10.13.
Company Overview
17.10.14.
Product Offerings
17.10.15.
Key Developments
17.10.16.
Financial Analysis
17.10.17.
SWOT Analysis
17.10.18.
Business Strategies
17.11.
Millar, Inc
17.11.12.
Company Details
17.11.13.
Company Overview
17.11.14.
Product Offerings
17.11.15.
Key Developments
17.11.16.
Financial Analysis
17.11.17.
SWOT Analysis
17.11.18.
Business Strategies
17.12.
Siemens Healthcare Private Limited
17.12.12.
Company Details
17.12.13.
Company Overview
17.12.14.
Product Offerings
17.12.15.
Key Developments
17.12.16.
Financial Analysis
17.12.17.
SWOT Analysis
17.12.18.
Business Strategies
17.13.
Transonic
17.13.12.
Company Details
17.13.13.
Company Overview
17.13.14.
Product Offerings
17.13.15.
Key Developments
17.13.16.
Financial Analysis
17.13.17.
SWOT Analysis
17.13.18.
Business Strategies
17.14.
Tyche MedTech, Inc.
17.14.12.
Company Details
17.14.13.
Company Overview
17.14.14.
Product Offerings
17.14.15.
Key Developments
17.14.16.
Financial Analysis
17.14.17.
SWOT Analysis
17.14.18.
Business Strategies
17.15.
Vanguard AG
17.15.12.
Company Details
17.15.13.
Company Overview
17.15.14.
Product Offerings
17.15.15.
Key Developments
17.15.16.
Financial Analysis
17.15.17.
SWOT Analysis
17.15.18.
Business Strategies
17.16.
World Precision Instruments
17.16.12.
Company Details
17.16.13.
Company Overview
17.16.14.
Product Offerings
17.16.15.
Key Developments
17.16.16.
Financial Analysis
17.16.17.
SWOT Analysis
17.16.18.
Business Strategies
17.17.
Zeus Industrial Products, Inc
17.17.12.
Company Details
17.17.13.
Company Overview
17.17.14.
Product Offerings
17.17.15.
Key Developments
17.17.16.
Financial Analysis
17.17.17.
SWOT Analysis
17.17.18.
Business Strategies
17.18.
Other Market Participants
18 Key Findings
Note: This ToC is tentative and can
be changed according to the research study conducted during the course of
report completion.
**Exclusive for Multi-User and
Enterprise User.
At Absolute Markets Insights, we are engaged in building both global as well as country specific reports. As a result, the approach taken for deriving the estimation and forecast for a specific country is a bit unique and different in comparison to the global research studies. In this case, we not only study the concerned market factors & trends prevailing in a particular country (from secondary research) but we also tend to calculate the actual market size & forecast from the revenue generated from the market participants involved in manufacturing or distributing the any concerned product. These companies can also be service providers. For analyzing any country specifically, we do consider the growth factors prevailing under the states/cities/county for the same. For instance, if we are analyzing an industry specific to United States, we primarily need to study about the states present under the same(where the product/service has the highest growth). Similar analysis will be followed by other countries. Our scope of the report changes with different markets.
Our research study is mainly implement through a mix of both secondary and primary research. Various sources such as industry magazines, trade journals, and government websites and trade associations are reviewed for gathering precise data. Primary interviews are conducted to validate the market size derived from secondary research. Industry experts, major manufacturers and distributors are contacted for further validation purpose on the current market penetration and growth trends.
Prominent participants in our primary research process include:
- Key Opinion Leaders namely the CEOs, CSOs, VPs, purchasing managers, amongst others
- Research and development participants, distributors/suppliers and subject matter experts
Secondary Research includes data extracted from paid data sources:
- Reuters
- Factiva
- Bloomberg
- One Source
- Hoovers
Research Methodology
Key Inclusions

Why Absolute Markets Insights?
An effective strategy is the entity that influences a business to stand out of the crowd. An organization with a phenomenal strategy for success dependably has the edge over the rivals in the market. It offers the organizations a head start in planning their strategy. Absolute Market Insights is the new initiation in the industry that will furnish you with the lead your business needs. Absolute Market Insights is the best destination for your business intelligence and analytical solutions; essentially because our qualitative and quantitative sources of information are competent to give one-stop solutions. We inventively combine qualitative and quantitative research in accurate proportions to have the best report, which not only gives the most recent insights but also assists you to grow.

