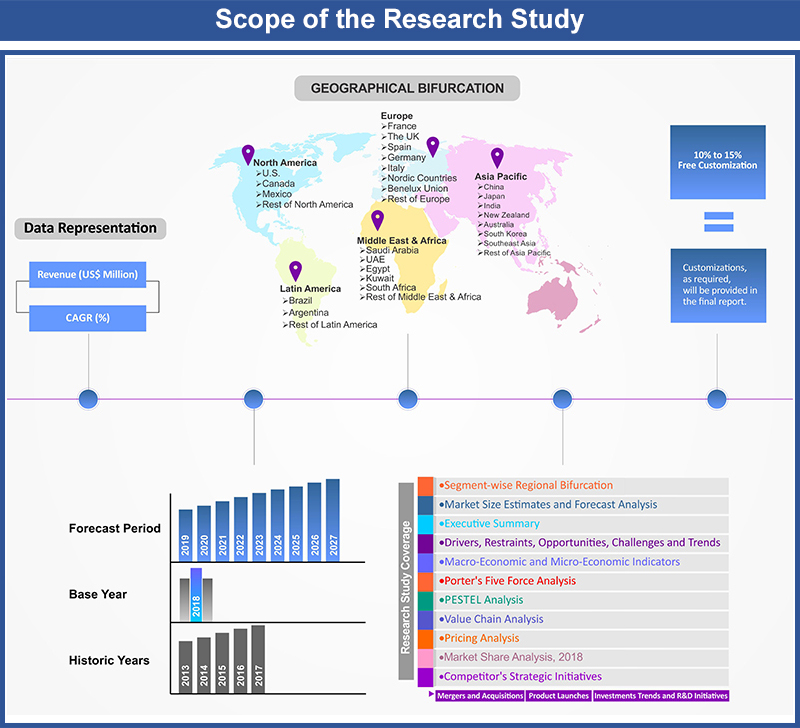
In Vitro Diagnostics Devices Market by Medical Devices (Reagents, Reagent Products, Calibrators, Control Materials, Kits, Instruments, Apparatus, Equipment and System); by Applications (Hospitals, Laboratories, Academic Institutes, Patient Self-Testing, Point-Of-Care Testing, Others); by Regional Outlook (U.S., Rest of North America, France, UK, Germany, Spain, Italy, Rest of Europe, China, Japan, India, Southeast Asia, Rest of Asia Pacific, GCC Countries, Southern Africa, Rest of MEA, Brazil, Rest of Latin America) – Global Insights, Growth, Size, Comparative Analysis, Trends and Forecast, 2018 - 2026
Industry Trends
In vitro diagnostics are tests conducted to determine infections, diseases or conditions. These tests are performed in laboratory, at professional health settings or are used by consumers at home. In-vitro diagnostics medical devices are accessories used for the examination of specimens derived from the human body to deliver information for diagnostics, monitoring or compatibility purposes. This would help to prevent disease, help to detect infection, monitor drug therapies and diagnose a medical condition. These tests could either be used alone or in combination. The devices used, vary from simple tests to sophisticated DNA technology that includes reagents, kits, calibrators, software, control materials and other instruments. Clinical chemistry, urine test strips, HIV tests or hepatitis, coagulation test systems, diabetic blood sugar monitoring systems, pregnancy tests, are few examples of in-vitro diagnostic medical devices. These could be used by doctors as well as by individuals at home. The global in vitro diagnostics devices market was valued at US$ 69.08 Bn in 2017 and is expected to reach US$ 90.86 Bn by 2022.
Global In Vitro Diagnostics Devices Market, By Region, 2018-2026 (US$ Billion)
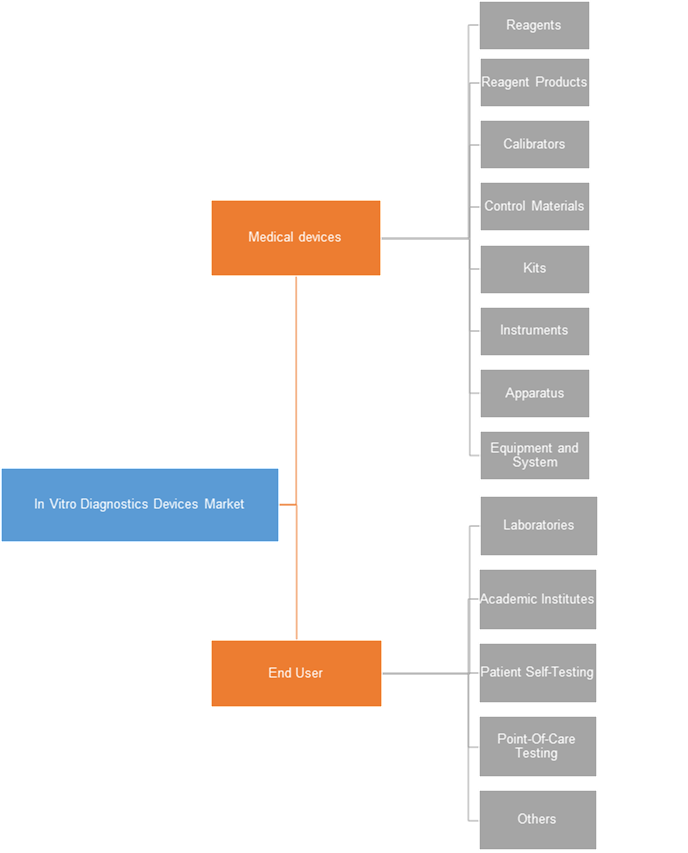
In Vitro Diagnostics Devices Market, By End User
On the basis of end user, laboratories held the largest market share. The expansion of healthcare industry has resulted in the emergence of a large number of laboratories offering various sampling tests. The automation of this processes ensures efficient and accurate results. This has led to the adoption of in vitro diagnostics devices in these laboratories. Laboratories are one of the major consumers utilizing in-vitro diagnostics devices on a large scale. The in vitro diagnostics devices applications include next generation sequencing tests, detecting genomic variations, etc. This segment is estimated to be the most attractive in the forecast period due to the increasing number of laboratories and the consequent wide spread adoption of in vitro diagnostics devices.
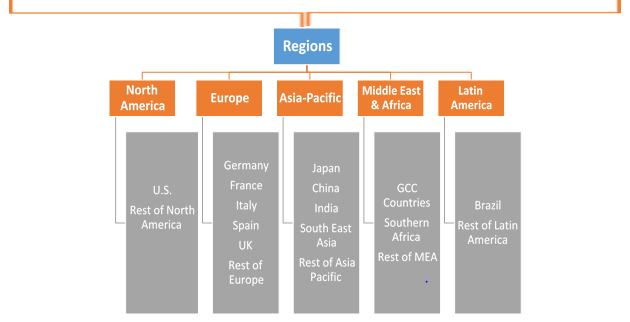
In Vitro Diagnostics Devices Market, By Region
North America is anticipated to display sustainable growth in in-vitro diagnostic devices market owing advantages such as, accessibility to technologies, and diagnostics opportunities in genetic testing and cancer screening. Moreover, technological advancements and strong research by the large number of market players such as Abbott Laboratories, Inc., Becton, Dickson and Company and Johnson & Johnson in North American region, are driving the market. Many companies are collaborating or acquiring small companies, which is significantly growing the market. For instance, in February 2016, Abbott
In Vitro Diagnostics Devices Industry Background
Laboratories, Inc., a global healthcare company acquired Alere. This transaction helped Abbott become one of the leading diagnostics provider of point of the care testing and offer flexible, new, cost-effective and high-quality products.
Competitive Market Share
The report provides both, qualitative and quantitative research of the market, as well as integrates worthy insights into the rational scenario and favored development methods adopted by the key contenders. The report also offers extensive research on the key players in this market and detailed insights on the competitiveness of these players. The key business strategies such as Mergers & Acquisitions (M&A), affiliations, collaborations, and contracts adopted by the major players are also recognized and analyzed in the report. For each company, the report recognizes their manufacturing base, competitors, product type, application and specification, pricing, and gross margin.
Some of the primary participants in global in vitro diagnostics market includes Abbott Laboratories, Inc., Becton, Dickson and Company, Johnson & Johnson, Beckman Coulter, Inc., bioMérieux SA, Bio-Rad Laboratories, Inc., Randox Laboratories Ltd., Roche Holding AG, Siemens AG, Sysmex Corporation, Thermo Fisher Scientific, amongst others.
In Vitro Diagnostics Devices Industry Background
1.
Introduction
1.1.
Market Scope
1.2.
Market Segmentation
1.3.
Methodology
1.4.
Assumptions
2.
In Vitro Diagnostics Devices
Market Snapshot
3.
Executive Summary: In Vitro
Diagnostics Devices Market
4.
Qualitative Analysis: In Vitro
Diagnostics Devices Market
4.1.
Introduction
4.1.1.
Product Definition
4.1.2.
Industry Development
4.2.
Market Dynamics
4.2.1.
Drivers
4.2.2.
Restraints
4.2.3.
Opportunities
4.3.
Trends in fMarket
5.
Global In Vitro Diagnostics
Devices Market Analysis and Forecasts, 2018 – 2026
5.1.
Overview
5.1.1.
Global Market Revenue (US$ Mn)
and Forecasts
5.2.
Global In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
5.2.1.
Reagents
5.2.1.1.
Definition
5.2.1.2.
Market Penetration
5.2.1.3.
Market Revenue Expected to
Increase by 2026
5.2.1.4.
Compound Annual Growth Rate
(CAGR)
5.2.2.
Reagent Products
5.2.2.1.
Definition
5.2.2.2.
Market Penetration
5.2.2.3.
Market Revenue Expected to
Increase by 2026
5.2.2.4.
Compound Annual Growth Rate
(CAGR)
5.2.3.
Calibrators
5.2.3.1.
Definition
5.2.3.2.
Market Penetration
5.2.3.3.
Market Revenue Expected to
Increase by 2026
5.2.3.4.
Compound Annual Growth Rate
(CAGR)
5.2.4.
Control Materials
5.2.4.1.
Definition
5.2.4.2.
Market Penetration
5.2.4.3.
Market Revenue Expected to
Increase by 2026
5.2.4.4.
Compound Annual Growth Rate
(CAGR)
5.2.5.
Kits
5.2.5.1.
Definition
5.2.5.2.
Market Penetration
5.2.5.3.
Market Revenue Expected to
Increase by 2026
5.2.5.4.
Compound Annual Growth Rate
(CAGR)
5.2.6.
Instruments
5.2.6.1.
Definition
5.2.6.2.
Market Penetration
5.2.6.3.
Market Revenue Expected to
Increase by 2026
5.2.6.4.
Compound Annual Growth Rate
(CAGR)
5.2.7.
Apparatus
5.2.7.1.
Definition
5.2.7.2.
Market Penetration
5.2.7.3.
Market Revenue Expected to
Increase by 2026
5.2.7.4.
Compound Annual Growth Rate
(CAGR)
5.2.8.
Equipment and System
5.2.8.1.
Definition
5.2.8.2.
Market Penetration
5.2.8.3.
Market Revenue Expected to Increase
by 2026
5.2.8.4.
Compound Annual Growth Rate
(CAGR)
5.3.
Key Segment for Channeling
Investments
5.3.1.
By Medical Devices
6.
Global In Vitro Diagnostics
Devices Market Analysis and Forecasts, 2018 – 2026
6.1.
Overview
6.2.
Global In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
6.2.1.
Laboratories
6.2.1.1.
Definition
6.2.1.2.
Market Penetration
6.2.1.3.
Market Revenue Expected to
Increase by 2026
6.2.1.4.
Compound Annual Growth Rate
(CAGR)
6.2.2.
Academic Institutes
6.2.2.1.
Definition
6.2.2.2.
Market Penetration
6.2.2.3.
Market Revenue Expected to
Increase by 2026
6.2.2.4.
Compound Annual Growth Rate
(CAGR)
6.2.3.
Patient Self-Testing
6.2.3.1.
Definition
6.2.3.2.
Market Penetration
6.2.3.3.
Market Revenue Expected to
Increase by 2026
6.2.3.4.
Compound Annual Growth Rate
(CAGR)
6.2.4.
Point-Of-Care Testing
6.2.4.1.
Definition
6.2.4.2.
Market Penetration
6.2.4.3.
Market Revenue Expected to
Increase by 2026
6.2.4.4.
Compound Annual Growth Rate
(CAGR)
6.2.5.
Others
6.2.5.1.
Definition
6.2.5.2.
Market Penetration
6.2.5.3.
Market Revenue Expected to
Increase by 2026
6.2.5.4.
Compound Annual Growth Rate
(CAGR)
6.3.
Key Segment for Channeling
Investments
6.3.1.
By End User
7.
North America In Vitro
Diagnostics Devices Market Analysis and Forecasts, 2018 – 2026
7.1.
Overview
7.1.1.
North America Market Revenue
(US$ Mn)
7.2.
North America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
7.2.1.
Reagents
7.2.2.
Reagent Products
7.2.3.
Calibrators
7.2.4.
Control Materials
7.2.5.
Kits
7.2.6.
Instruments
7.2.7.
Apparatus
7.2.8.
Equipment and System
7.3.
North America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
7.3.1.
Laboratories
7.3.2.
Academic Institutes
7.3.3.
Patient Self-Testing
7.3.4.
Point-Of-Care Testing
7.3.5.
Others
7.4.
North America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Country
7.4.1.
U.S.
7.4.1.1.
U.S. In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
7.4.1.1.1.
Reagents
7.4.1.1.2.
Reagent Products
7.4.1.1.3.
Calibrators
7.4.1.1.4.
Control Materials
7.4.1.1.5.
Kits
7.4.1.1.6.
Instruments
7.4.1.1.7.
Apparatus
7.4.1.1.8.
Equipment and System
7.4.1.2.
U.S. In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
7.4.1.2.1.
Laboratories
7.4.1.2.2.
Academic Institutes
7.4.1.2.3.
Patient Self-Testing
7.4.1.2.4.
Point-Of-Care Testing
7.4.1.2.5.
Others
7.4.2.
Rest of North America
7.4.2.1.
Rest of North America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
7.4.2.1.1.
Reagents
7.4.2.1.2.
Reagent Products
7.4.2.1.3.
Calibrators
7.4.2.1.4.
Control Materials
7.4.2.1.5.
Kits
7.4.2.1.6.
Instruments
7.4.2.1.7.
Apparatus
7.4.2.1.8.
Equipment and System
7.4.2.2.
Rest of North America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
7.4.2.2.1.
Laboratories
7.4.2.2.2.
Academic Institutes
7.4.2.2.3.
Patient Self-Testing
7.4.2.2.4.
Point-Of-Care Testing
7.4.2.2.5.
Others
7.5.
Key Segment for Channeling
Investments
7.5.1.
By Country
7.5.2.
By Medical Devices
7.5.3.
By End User
8.
Europe In Vitro Diagnostics
Devices Market Analysis and Forecasts, 2018 – 2026
8.1.
Overview
8.1.1.
Europe Market Revenue (US$ Mn)
8.2.
Europe In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.2.1.
Reagents
8.2.2.
Reagent Products
8.2.3.
Calibrators
8.2.4.
Control Materials
8.2.5.
Kits
8.2.6.
Instruments
8.2.7.
Apparatus
8.2.8.
Equipment and System
8.3.
Europe In Vitro Diagnostics Devices
Market Revenue (US$ Mn) and Forecasts, By End User
8.3.1.
Laboratories
8.3.2.
Academic Institutes
8.3.3.
Patient Self-Testing
8.3.4.
Point-Of-Care Testing
8.3.5.
Others
8.4.
Europe In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Country
8.4.1.
France
8.4.1.1.
France In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.1.1.1.
Reagents
8.4.1.1.2.
Reagent Products
8.4.1.1.3.
Calibrators
8.4.1.1.4.
Control Materials
8.4.1.1.5.
Kits
8.4.1.1.6.
Instruments
8.4.1.1.7.
Apparatus
8.4.1.1.8.
Equipment and System
8.4.1.2.
France In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.1.2.1.
Laboratories
8.4.1.2.2.
Academic Institutes
8.4.1.2.3.
Patient Self-Testing
8.4.1.2.4.
Point-Of-Care Testing
8.4.1.2.5.
Others
8.4.2.
The UK
8.4.2.1.
The UK In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.2.1.1.
Reagents
8.4.2.1.2.
Reagent Products
8.4.2.1.3.
Calibrators
8.4.2.1.4.
Control Materials
8.4.2.1.5.
Kits
8.4.2.1.6.
Instruments
8.4.2.1.7.
Apparatus
8.4.2.1.8.
Equipment and System
8.4.2.2.
The UK In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.2.2.1.
Laboratories
8.4.2.2.2.
Academic Institutes
8.4.2.2.3.
Patient Self-Testing
8.4.2.2.4.
Point-Of-Care Testing
8.4.2.2.5.
Others
8.4.3.
Spain
8.4.3.1.
Spain In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.3.1.1.
Reagents
8.4.3.1.2.
Reagent Products
8.4.3.1.3.
Calibrators
8.4.3.1.4.
Control Materials
8.4.3.1.5.
Kits
8.4.3.1.6.
Instruments
8.4.3.1.7.
Apparatus
8.4.3.1.8.
Equipment and System
8.4.3.2.
Spain In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.3.2.1.
Laboratories
8.4.3.2.2.
Academic Institutes
8.4.3.2.3.
Patient Self-Testing
8.4.3.2.4.
Point-Of-Care Testing
8.4.3.2.5.
Others
8.4.4.
Germany
8.4.4.1.
Germany In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.4.1.1.
Reagents
8.4.4.1.2.
Reagent Products
8.4.4.1.3.
Calibrators
8.4.4.1.4.
Control Materials
8.4.4.1.5.
Kits
8.4.4.1.6.
Instruments
8.4.4.1.7.
Apparatus
8.4.4.1.8.
Equipment and System
8.4.4.2.
Germany In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.4.2.1.
Laboratories
8.4.4.2.2.
Academic Institutes
8.4.4.2.3.
Patient Self-Testing
8.4.4.2.4.
Point-Of-Care Testing
8.4.4.2.5.
Others
8.4.5.
Italy
8.4.5.1.
Italy In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.5.1.1.
Reagents
8.4.5.1.2.
Reagent Products
8.4.5.1.3.
Calibrators
8.4.5.1.4.
Control Materials
8.4.5.1.5.
Kits
8.4.5.1.6.
Instruments
8.4.5.1.7.
Apparatus
8.4.5.1.8.
Equipment and System
8.4.5.2.
Italy In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.5.2.1.
Laboratories
8.4.5.2.2.
Academic Institutes
8.4.5.2.3.
Patient Self-Testing
8.4.5.2.4.
Point-Of-Care Testing
8.4.5.2.5.
Others
8.4.6.
Rest of Europe
8.4.6.1.
Rest of Europe In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
8.4.6.1.1.
Reagents
8.4.6.1.2.
Reagent Products
8.4.6.1.3.
Calibrators
8.4.6.1.4.
Control Materials
8.4.6.1.5.
Kits
8.4.6.1.6.
Instruments
8.4.6.1.7.
Apparatus
8.4.6.1.8.
Equipment and System
8.4.6.2.
Rest of Europe In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
8.4.6.2.1.
Laboratories
8.4.6.2.2.
Academic Institutes
8.4.6.2.3.
Patient Self-Testing
8.4.6.2.4.
Point-Of-Care Testing
8.4.6.2.5.
Others
8.5.
Key Segment for Channeling
Investments
8.5.1.
By Country
8.5.2.
By Medical Devices
8.5.3.
By End User
9.
Asia Pacific In Vitro Diagnostics Devices Market Analysis
and Forecasts, 2018 – 2026
9.1.
Overview
9.1.1.
Asia Pacific Market Revenue (US$
Mn)
9.2.
Asia Pacific In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.2.1.
Reagents
9.2.2.
Reagent Products
9.2.3.
Calibrators
9.2.4.
Control Materials
9.2.5.
Kits
9.2.6.
Instruments
9.2.7.
Apparatus
9.2.8.
Equipment and System
9.3.
Asia Pacific In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.3.1.
Laboratories
9.3.2.
Academic Institutes
9.3.3.
Patient Self-Testing
9.3.4.
Point-Of-Care Testing
9.3.5.
Others
9.4.
Asia Pacific In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Country
9.4.1.
China
9.4.1.1.
China In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.4.1.1.1.
Reagents
9.4.1.1.2.
Reagent Products
9.4.1.1.3.
Calibrators
9.4.1.1.4.
Control Materials
9.4.1.1.5.
Kits
9.4.1.1.6.
Instruments
9.4.1.1.7.
Apparatus
9.4.1.1.8.
Equipment and System
9.4.1.2.
China In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.4.1.2.1.
Laboratories
9.4.1.2.2.
Academic Institutes
9.4.1.2.3.
Patient Self-Testing
9.4.1.2.4.
Point-Of-Care Testing
9.4.1.2.5.
Others
9.4.2.
Japan
9.4.2.1.
Japan In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.4.2.1.1.
Reagents
9.4.2.1.2.
Reagent Products
9.4.2.1.3.
Calibrators
9.4.2.1.4.
Control Materials
9.4.2.1.5.
Kits
9.4.2.1.6.
Instruments
9.4.2.1.7.
Apparatus
9.4.2.1.8.
Equipment and System
9.4.2.2.
Japan In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.4.2.2.1.
Laboratories
9.4.2.2.2.
Academic Institutes
9.4.2.2.3.
Patient Self-Testing
9.4.2.2.4.
Point-Of-Care Testing
9.4.2.2.5.
Others
9.4.3.
India
9.4.3.1.
India In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.4.3.1.1.
Reagents
9.4.3.1.2.
Reagent Products
9.4.3.1.3.
Calibrators
9.4.3.1.4.
Control Materials
9.4.3.1.5.
Kits
9.4.3.1.6.
Instruments
9.4.3.1.7.
Apparatus
9.4.3.1.8.
Equipment and System
9.4.3.2.
India In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.4.3.2.1.
Laboratories
9.4.3.2.2.
Academic Institutes
9.4.3.2.3.
Patient Self-Testing
9.4.3.2.4.
Point-Of-Care Testing
9.4.3.2.5.
Others
9.4.4.
Southeast Asia
9.4.4.1.
Southeast Asia In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.4.4.1.1.
Reagents
9.4.4.1.2.
Reagent Products
9.4.4.1.3.
Calibrators
9.4.4.1.4.
Control Materials
9.4.4.1.5.
Kits
9.4.4.1.6.
Instruments
9.4.4.1.7.
Apparatus
9.4.4.1.8.
Equipment and System
9.4.4.2.
Southeast Asia In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.4.4.2.1.
Laboratories
9.4.4.2.2.
Academic Institutes
9.4.4.2.3.
Patient Self-Testing
9.4.4.2.4.
Point-Of-Care Testing
9.4.4.2.5.
Others
9.4.5.
Rest of Asia Pacific
9.4.5.1.
Rest of Asia Pacific In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
9.4.5.1.1.
Reagents
9.4.5.1.2.
Reagent Products
9.4.5.1.3.
Calibrators
9.4.5.1.4.
Control Materials
9.4.5.1.5.
Kits
9.4.5.1.6.
Instruments
9.4.5.1.7.
Apparatus
9.4.5.1.8.
Equipment and System
9.4.5.2.
Rest of Asia Pacific In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
9.4.5.2.1.
Laboratories
9.4.5.2.2.
Academic Institutes
9.4.5.2.3.
Patient Self-Testing
9.4.5.2.4.
Point-Of-Care Testing
9.4.5.2.5.
Others
9.5.
Key Segment for Channeling
Investments
9.5.1.
By Country
9.5.2.
By Medical Devices
9.5.3.
By End User
10. Middle East and Africa
In Vitro Diagnostics Devices Market Analysis and Forecasts, 2018 – 2026
10.1. Overview
10.1.1. Middle East and Africa Market Revenue (US$ Mn)
10.2. Middle East and Africa In Vitro Diagnostics Devices Market
Revenue (US$ Mn) and Forecasts, By Medical Devices
10.2.1. Reagents
10.2.2. Reagent Products
10.2.3. Calibrators
10.2.4. Control Materials
10.2.5. Kits
10.2.6. Instruments
10.2.7. Apparatus
10.2.8. Equipment and System
10.3. Middle East and Africa In Vitro Diagnostics Devices Market
Revenue (US$ Mn) and Forecasts, By End User
10.3.1. Laboratories
10.3.2. Academic Institutes
10.3.3. Patient Self-Testing
10.3.4. Point-Of-Care Testing
10.3.5. Others
10.4. Middle East and Africa In Vitro Diagnostics Devices Market
Revenue (US$ Mn) and Forecasts, By Country
10.4.1. GCC Countries
10.4.1.1.
GCC Countries In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
10.4.1.1.1.
Reagents
10.4.1.1.2.
Reagent Products
10.4.1.1.3.
Calibrators
10.4.1.1.4.
Control Materials
10.4.1.1.5.
Kits
10.4.1.1.6.
Instruments
10.4.1.1.7.
Apparatus
10.4.1.1.8.
Equipment and System
10.4.1.2.
GCC Countries In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
10.4.1.2.1.
Laboratories
10.4.1.2.2.
Academic Institutes
10.4.1.2.3.
Patient Self-Testing
10.4.1.2.4.
Point-Of-Care Testing
10.4.1.2.5.
Others
10.4.2. Southern Africa
10.4.2.1.
Southern Africa In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
10.4.2.1.1.
Reagents
10.4.2.1.2.
Reagent Products
10.4.2.1.3.
Calibrators
10.4.2.1.4.
Control Materials
10.4.2.1.5.
Kits
10.4.2.1.6.
Instruments
10.4.2.1.7.
Apparatus
10.4.2.1.8.
Equipment and System
10.4.2.2.
Southern Africa In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
10.4.2.2.1.
Laboratories
10.4.2.2.2.
Academic Institutes
10.4.2.2.3.
Patient Self-Testing
10.4.2.2.4.
Point-Of-Care Testing
10.4.2.2.5.
Others
10.4.3. Rest of MEA
10.4.3.1.
Rest of MEA In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
10.4.3.1.1.
Reagents
10.4.3.1.2.
Reagent Products
10.4.3.1.3.
Calibrators
10.4.3.1.4.
Control Materials
10.4.3.1.5.
Kits
10.4.3.1.6.
Instruments
10.4.3.1.7.
Apparatus
10.4.3.1.8.
Equipment and System
10.4.3.2.
Rest of MEA In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
10.4.3.2.1.
Laboratories
10.4.3.2.2.
Academic Institutes
10.4.3.2.3.
Patient Self-Testing
10.4.3.2.4.
Point-Of-Care Testing
10.4.3.2.5.
Others
10.5. Key Segment for Channeling Investments
10.5.1. By Country
10.5.2. By Medical Devices
10.5.3. By End User
11. Latin America In Vitro Diagnostics Devices Market Analysis
and Forecasts, 2018 – 2026
11.1. Overview
11.1.1. Latin America In Vitro Diagnostics Devices Market Revenue (US$
Mn)
11.2. Latin America In Vitro Diagnostics Devices Market Revenue (US$
Mn) and Forecasts, By Medical Devices
11.2.1. Reagents
11.2.2. Reagent Products
11.2.3. Calibrators
11.2.4. Control Materials
11.2.5. Kits
11.2.6. Instruments
11.2.7. Apparatus
11.2.8. Equipment and System
11.3. Latin America In Vitro Diagnostics Devices Market Revenue (US$
Mn) and Forecasts, By End User
11.3.1. Laboratories
11.3.2. Academic Institutes
11.3.3. Patient Self-Testing
11.3.4. Point-Of-Care Testing
11.3.5. Others
11.4. Latin America In Vitro Diagnostics Devices Market Revenue (US$
Mn) and Forecasts, By Country
11.4.1. Brazil
11.4.1.1.
Brazil In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
11.4.1.1.1.
Reagents
11.4.1.1.2.
Reagent Products
11.4.1.1.3.
Calibrators
11.4.1.1.4.
Control Materials
11.4.1.1.5.
Kits
11.4.1.1.6.
Instruments
11.4.1.1.7.
Apparatus
11.4.1.1.8.
Equipment and System
11.4.1.2.
Brazil In Vitro Diagnostics
Devices Market Revenue (US$ Mn) and Forecasts, By End User
11.4.1.2.1.
Laboratories
11.4.1.2.2.
Academic Institutes
11.4.1.2.3.
Patient Self-Testing
11.4.1.2.4.
Point-Of-Care Testing
11.4.1.2.5.
Others
11.4.2. Rest of Latin America
11.4.2.1.
Rest of Latin America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By Medical Devices
11.4.2.1.1.
Reagents
11.4.2.1.2.
Reagent Products
11.4.2.1.3.
Calibrators
11.4.2.1.4.
Control Materials
11.4.2.1.5.
Kits
11.4.2.1.6.
Instruments
11.4.2.1.7.
Apparatus
11.4.2.1.8.
Equipment and System
11.4.2.2.
Rest of Latin America In Vitro
Diagnostics Devices Market Revenue (US$ Mn) and Forecasts, By End User
11.4.2.2.1.
Laboratories
11.4.2.2.2.
Academic Institutes
11.4.2.2.3.
Patient Self-Testing
11.4.2.2.4.
Point-Of-Care Testing
11.4.2.2.5.
Others
11.5. Key Segment for Channeling Investments
11.5.1. By Country
11.5.2. By Medical Devices
11.5.3. By End User
12. Competitive Benchmarking
12.1. Player Positioning Analysis
12.2. Global Presence and Growth Strategies
13. Player Profiles
13.1. Abbott Laboratories, Inc.
13.1.1. Company Details
13.1.2. Company Overview
13.1.3. Product Offerings
13.1.4. Key Developments
13.1.5. Financial Analysis
13.1.6. SWOT Analysis
13.1.7. Business Strategies
13.2. Becton, Dickson & Company
13.2.1. Company Details
13.2.2. Company Overview
13.2.3. Product Offerings
13.2.4. Key Developments
13.2.5. Financial Analysis
13.2.6. SWOT Analysis
13.2.7. Business Strategies
13.3. Johnson & Johnson
13.3.1. Company Details
13.3.2. Company Overview
13.3.3. Product Offerings
13.3.4. Key Developments
13.3.5. Financial Analysis
13.3.6. SWOT Analysis
13.3.7. Business Strategies
13.4. Beckman Coulter, Inc.
13.4.1. Company Details
13.4.2. Company Overview
13.4.3. Product Offerings
13.4.4. Key Developments
13.4.5. Financial Analysis
13.4.6. SWOT Analysis
13.4.7. Business Strategies
13.5. bioMérieux SA
13.5.1. Company Details
13.5.2. Company Overview
13.5.3. Product Offerings
13.5.4. Key Developments
13.5.5. Financial Analysis
13.5.6. SWOT Analysis
13.5.7. Business Strategies
13.6. Bio-Rad Laboratories, Inc.
13.6.1. Company Details
13.6.2. Company Overview
13.6.3. Product Offerings
13.6.4. Key Developments
13.6.5. Financial Analysis
13.6.6. SWOT Analysis
13.6.7. Business Strategies
13.7. Randox Laboratories Ltd.
13.7.1. Company Details
13.7.2. Company Overview
13.7.3. Product Offerings
13.7.4. Key Developments
13.7.5. Financial Analysis
13.7.6. SWOT Analysis
13.7.7. Business Strategies
13.8. Roche Holding AG
13.8.1. Company Details
13.8.2. Company Overview
13.8.3. Product Offerings
13.8.4. Key Developments
13.8.5. Financial Analysis
13.8.6. SWOT Analysis
13.8.7. Business Strategies
13.9. Siemens AG
13.9.1. Company Details
13.9.2. Company Overview
13.9.3. Product Offerings
13.9.4. Key Developments
13.9.5. Financial Analysis
13.9.6. SWOT Analysis
13.9.7. Business Strategies
13.10.
Sysmex Corporation
13.10.1.
Company Details
13.10.2.
Company Overview
13.10.3.
Product Offerings
13.10.4.
Key Developments
13.10.5.
Financial Analysis
13.10.6.
SWOT Analysis
13.10.7.
Business Strategies
13.11. Thermo Fisher Scientific
13.11.1.
Company Details
13.11.2.
Company Overview
13.11.3.
Product Offerings
13.11.4.
Key Developments
13.11.5.
Financial Analysis
13.11.6.
SWOT Analysis
13.11.7.
Business Strategies
Note: This ToC is tentative and can be changed according to the research
study conducted during the course of report completion.
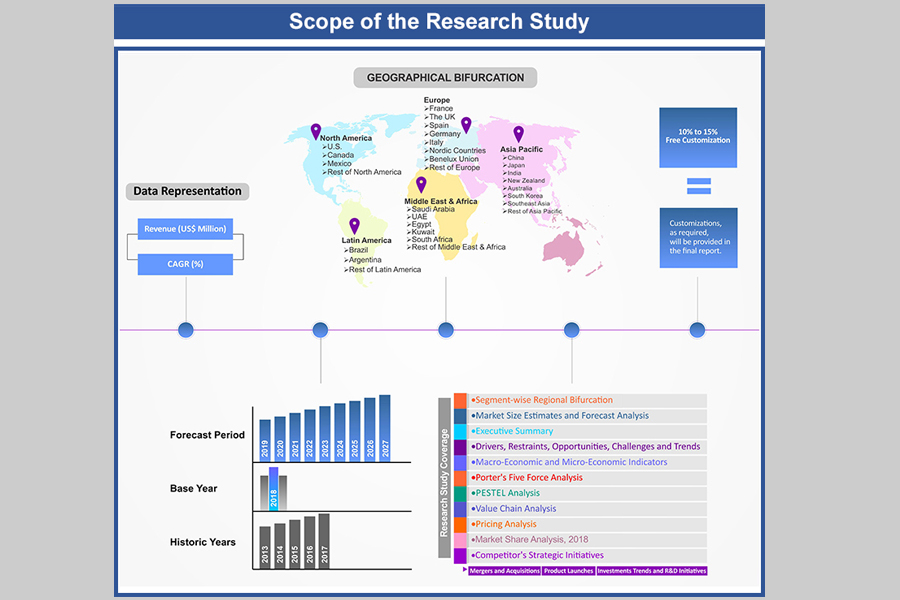
At Absolute Markets Insights, we are engaged in building both global as well as country specific reports. As a result, the approach taken for deriving the estimation and forecast for a specific country is a bit unique and different in comparison to the global research studies. In this case, we not only study the concerned market factors & trends prevailing in a particular country (from secondary research) but we also tend to calculate the actual market size & forecast from the revenue generated from the market participants involved in manufacturing or distributing the any concerned product. These companies can also be service providers. For analyzing any country specifically, we do consider the growth factors prevailing under the states/cities/county for the same. For instance, if we are analyzing an industry specific to United States, we primarily need to study about the states present under the same(where the product/service has the highest growth). Similar analysis will be followed by other countries. Our scope of the report changes with different markets.
Our research study is mainly implement through a mix of both secondary and primary research. Various sources such as industry magazines, trade journals, and government websites and trade associations are reviewed for gathering precise data. Primary interviews are conducted to validate the market size derived from secondary research. Industry experts, major manufacturers and distributors are contacted for further validation purpose on the current market penetration and growth trends.
Prominent participants in our primary research process include:
- Key Opinion Leaders namely the CEOs, CSOs, VPs, purchasing managers, amongst others
- Research and development participants, distributors/suppliers and subject matter experts
Secondary Research includes data extracted from paid data sources:
- Reuters
- Factiva
- Bloomberg
- One Source
- Hoovers
Research Methodology
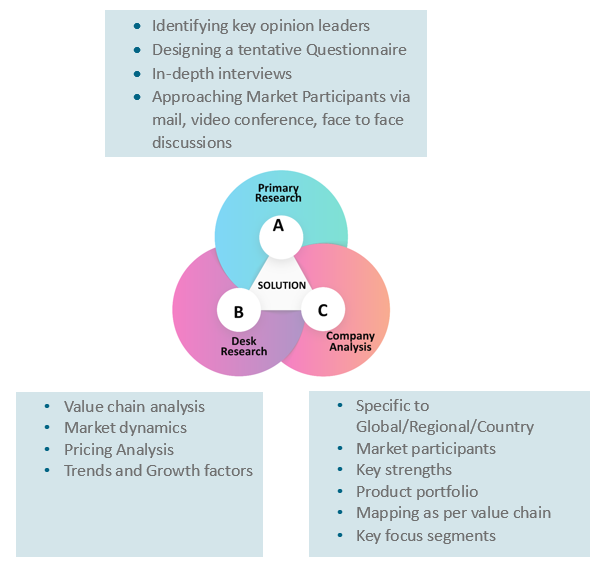
Key Inclusions

Reach to us
Call us on
+91-74002-42424
Drop us an email at
sales@absolutemarketsinsights.com
Why Absolute Markets Insights?
An effective strategy is the entity that influences a business to stand out of the crowd. An organization with a phenomenal strategy for success dependably has the edge over the rivals in the market. It offers the organizations a head start in planning their strategy. Absolute Market Insights is the new initiation in the industry that will furnish you with the lead your business needs. Absolute Market Insights is the best destination for your business intelligence and analytical solutions; essentially because our qualitative and quantitative sources of information are competent to give one-stop solutions. We inventively combine qualitative and quantitative research in accurate proportions to have the best report, which not only gives the most recent insights but also assists you to grow.